Antigen-specific CD4(+) T cells regulate function of myeloid-derived suppressor cells in cancer via retrograde MHC class II signaling
- PMID: 22237629
- PMCID: PMC4062074
- DOI: 10.1158/0008-5472.CAN-11-2863
Antigen-specific CD4(+) T cells regulate function of myeloid-derived suppressor cells in cancer via retrograde MHC class II signaling
Abstract
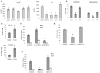
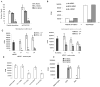
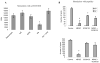
Myeloid-derived suppressor cells (MDSC) play a major role in cancer-related immune suppression, yet the nature of this suppression remains controversial. In this study, we evaluated the ability of MDSCs to elicit CD4(+) T-cell tolerance in different mouse tumor models. In contrast to CD8(+) T-cell tolerance, which could be induced by MDSCs in all the tumor models tested, CD4(+) T-cell tolerance could be elicited in only one of the models (MC38) in which a substantial level of MHC class II was expressed on MDSCs compared with control myeloid cells. Mechanistic investigations revealed that MDSCs deficient in MHC class II could induce tolerance to CD8(+) T cells but not to CD4(+) T cells. Unexpectedly, antigen-specific CD4(+) T cells (but not CD8(+) T cells) could dramatically enhance the immune suppressive activity of MDSCs by converting them into powerful nonspecific suppressor cells. This striking effect was mediated by direct cell-cell contact through cross-linking of MHC class II on MDSCs. We also implicated an Ets-1 transcription factor-regulated increase in expression of Cox-2 and prostaglandin E2 in MDSCs in mediating this effect. Together, our findings suggest that activated CD4(+) T cells that are antigen specific may enhance the immune suppressive activity of MDSCs, a mechanism that might serve normally as a negative feedback loop to control immune responses that becomes dysregulated in cancer.
Figures


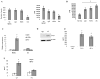

Similar articles
-
Autophagy orchestrates the regulatory program of tumor-associated myeloid-derived suppressor cells.J Clin Invest. 2018 Aug 31;128(9):3840-3852. doi: 10.1172/JCI120888. Epub 2018 Aug 6. J Clin Invest. 2018. PMID: 29920188 Free PMC article.
-
Myeloid-derived suppressor cell functionality and interaction with Leishmania major parasites differ in C57BL/6 and BALB/c mice.Eur J Immunol. 2014 Nov;44(11):3295-306. doi: 10.1002/eji.201344335. Epub 2014 Sep 19. Eur J Immunol. 2014. PMID: 25142017
-
Increased numbers and suppressive activity of regulatory CD25(+)CD4(+) T lymphocytes in the absence of CD4 engagement by MHC class II molecules.Cell Immunol. 2013 Apr;282(2):117-28. doi: 10.1016/j.cellimm.2013.05.002. Epub 2013 May 15. Cell Immunol. 2013. PMID: 23770721
-
Regulation of suppressive function of myeloid-derived suppressor cells by CD4+ T cells.Semin Cancer Biol. 2012 Aug;22(4):282-8. doi: 10.1016/j.semcancer.2012.01.010. Epub 2012 Jan 31. Semin Cancer Biol. 2012. PMID: 22313876 Free PMC article. Review.
-
Myeloid derived suppressor cells and their role in tolerance induction in cancer.J Dermatol Sci. 2010 Jul;59(1):1-6. doi: 10.1016/j.jdermsci.2010.05.001. J Dermatol Sci. 2010. PMID: 20570112 Review.
Cited by
-
Reciprocal relationship between myeloid-derived suppressor cells and T cells.J Immunol. 2013 Jul 1;191(1):17-23. doi: 10.4049/jimmunol.1300654. J Immunol. 2013. PMID: 23794702 Free PMC article. Review.
-
Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected.J Clin Invest. 2015 Sep;125(9):3356-64. doi: 10.1172/JCI80005. Epub 2015 Jul 13. J Clin Invest. 2015. PMID: 26168215 Free PMC article. Review.
-
Frequencies of circulating MDSC correlate with clinical outcome of melanoma patients treated with ipilimumab.Cancer Immunol Immunother. 2014 Mar;63(3):247-57. doi: 10.1007/s00262-013-1508-5. Epub 2013 Dec 20. Cancer Immunol Immunother. 2014. PMID: 24357148 Free PMC article.
-
Hepatitis C Virus Induces MDSCs-Like Monocytes through TLR2/PI3K/AKT/STAT3 Signaling.PLoS One. 2017 Jan 23;12(1):e0170516. doi: 10.1371/journal.pone.0170516. eCollection 2017. PLoS One. 2017. PMID: 28114346 Free PMC article.
-
Donor myeloid derived suppressor cells (MDSCs) prolong allogeneic cardiac graft survival through programming of recipient myeloid cells in vivo.Sci Rep. 2020 Aug 28;10(1):14249. doi: 10.1038/s41598-020-71289-z. Sci Rep. 2020. PMID: 32859934 Free PMC article.
References
-
- Gabrilovich D, Hurwitz A. Tumor induced immune suppression. New York: Springer; 2008.
-
- Solito S, Bronte V, Mandruzzato S. Antigen specificity of immune suppression by myeloid-derived suppressor cells. J Leukoc Biol. 2010 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

