Synaptic plasticity in the medial superior olive of hearing, deaf, and cochlear-implanted cats
- PMID: 22237661
- PMCID: PMC3963361
- DOI: 10.1002/cne.23038
Synaptic plasticity in the medial superior olive of hearing, deaf, and cochlear-implanted cats
Abstract
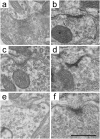
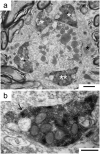
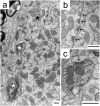
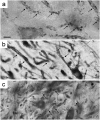
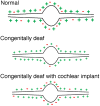
The medial superior olive (MSO) is a key auditory brainstem structure that receives binaural inputs and is implicated in processing interaural time disparities used for sound localization. The deaf white cat, a proven model of congenital deafness, was used to examine how deafness and cochlear implantation affected the synaptic organization at this binaural center in the ascending auditory pathway. The patterns of axosomatic and axodendritic organization were determined for principal neurons from the MSO of hearing, deaf, and deaf cats with cochlear implants. The nature of the synapses was evaluated through electron microscopy, ultrastructure analysis of the synaptic vesicles, and immunohistochemistry. The results show that the proportion of inhibitory axosomatic terminals was significantly smaller in deaf animals when compared with hearing animals. However, after a period of electrical stimulation via cochlear implants the proportion of inhibitory inputs resembled that of hearing animals. Additionally, the excitatory axodendritic boutons of hearing cats were found to be significantly larger than those of deaf cats. Boutons of stimulated cats were significantly larger than the boutons in deaf cats, although not as large as in the hearing cats, indicating a partial recovery of excitatory inputs to MSO dendrites after stimulation. These results exemplify dynamic plasticity in the auditory brainstem and reveal that electrical stimulation through cochlear implants has a restorative effect on synaptic organization in the MSO.
Copyright © 2012 Wiley Periodicals, Inc.
Figures



Similar articles
-
Bilateral effects of unilateral cochlear implantation in congenitally deaf cats.J Comp Neurol. 2010 Jun 15;518(12):2382-404. doi: 10.1002/cne.22339. J Comp Neurol. 2010. PMID: 20437534 Free PMC article.
-
Ultrastructural transynaptic effects of unilateral cochlear ablation in the gerbil medial superior olive.Hear Res. 2002 Nov;173(1-2):43-61. doi: 10.1016/s0378-5955(02)00606-8. Hear Res. 2002. PMID: 12372634
-
Synaptic plasticity after chemical deafening and electrical stimulation of the auditory nerve in cats.J Comp Neurol. 2010 Apr 1;518(7):1046-63. doi: 10.1002/cne.22262. J Comp Neurol. 2010. PMID: 20127807 Free PMC article.
-
Use it or lose it? Lessons learned from the developing brains of children who are deaf and use cochlear implants to hear.Brain Topogr. 2011 Oct;24(3-4):204-19. doi: 10.1007/s10548-011-0181-2. Epub 2011 Apr 11. Brain Topogr. 2011. PMID: 21479928 Review.
-
What is the optimal timing for bilateral cochlear implantation in children?Cochlear Implants Int. 2011 Aug;12 Suppl 2:S8-14. doi: 10.1179/146701011X13074645127199. Cochlear Implants Int. 2011. PMID: 21917210 Review.
Cited by
-
Commentary: "Compensatory plasticity: time matters".Front Neurosci. 2015 Oct 8;9:348. doi: 10.3389/fnins.2015.00348. eCollection 2015. Front Neurosci. 2015. PMID: 26500477 Free PMC article. No abstract available.
-
Multisensory training improves auditory spatial processing following bilateral cochlear implantation.J Neurosci. 2014 Aug 13;34(33):11119-30. doi: 10.1523/JNEUROSCI.4767-13.2014. J Neurosci. 2014. PMID: 25122908 Free PMC article.
-
Relationship between Metabolomics Profile of Perilymph in Cochlear-Implanted Patients and Duration of Hearing Loss.Metabolites. 2019 Nov 1;9(11):262. doi: 10.3390/metabo9110262. Metabolites. 2019. PMID: 31683919 Free PMC article.
-
Congenital and prolonged adult-onset deafness cause distinct degradations in neural ITD coding with bilateral cochlear implants.J Assoc Res Otolaryngol. 2013 Jun;14(3):393-411. doi: 10.1007/s10162-013-0380-5. Epub 2013 Mar 5. J Assoc Res Otolaryngol. 2013. PMID: 23462803 Free PMC article.
-
Microsecond interaural time difference discrimination restored by cochlear implants after neonatal deafness.Elife. 2021 Jan 11;10:e59300. doi: 10.7554/eLife.59300. Elife. 2021. PMID: 33427644 Free PMC article.
References
-
- Atwood HL, Lang F, Morin WA. Synaptic vesicles: selective depletion in crayfish excitatory and inhibitory axons. Science. 1972;176:1353–1355. - PubMed
-
- Babalian AL, Ryugo DK, Rouiller EM. Discharge properties of identified cochlear nucleus neurons and auditory nerve fibers in response to repetitive electrical stimulation of the auditory nerve. Exp Brain Res. 2003;153:452–460. - PubMed
-
- Barrett TW. Superposition of binaural influences on single neuron activity in the medial superior olive elicited by electrical stimulation of the osseous spiral laminae. Brain Res Bull. 1976;1:209–228. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

