Addition of insulin glargine or NPH insulin to metformin monotherapy in poorly controlled type 2 diabetic patients decreases IGF-I bioactivity similarly
- PMID: 22237688
- PMCID: PMC3296010
- DOI: 10.1007/s00125-011-2435-7
Addition of insulin glargine or NPH insulin to metformin monotherapy in poorly controlled type 2 diabetic patients decreases IGF-I bioactivity similarly
Abstract
Aims/hypothesis: The aim of this study was to compare IGF-I bioactivity 36 weeks after the addition of insulin glargine (A21Gly,B31Arg,B32Arg human insulin) or NPH insulin to metformin therapy in type 2 diabetic patients who had poor glucose control under metformin monotherapy.
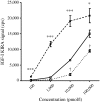
Methods: In the Lantus plus Metformin (LANMET) study, 110 poorly controlled insulin-naive type 2 diabetic patients were randomised to receive metformin with either insulin glargine (G+MET) or NPH insulin (NPH+MET). In the present study, IGF-I bioactivity was measured, retrospectively, in 104 out of the 110 initially included LANMET participants before and after 36 weeks of insulin therapy. IGF-I bioactivity was measured using an IGF-I kinase receptor activation assay.
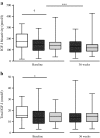
Results: After 36 weeks of insulin therapy, insulin doses were comparable between the G+MET (68 ± 5.7 U/day) and NPH+MET (71 ± 6.2 U/day) groups (p = 0.68). Before insulin therapy, circulating IGF-I bioactivity was similar between the G+MET (134 ± 9 pmol/l) and NPH+MET (135 ± 10 pmol/l) groups (p = 0.83). After 36 weeks, IGF-I bioactivity had decreased significantly (p = 0.001) and did not differ between the G+MET (116 ± 9 pmol/l) and NPH+MET (117 ± 10 pmol/l) groups (p = 0.91). At baseline and after insulin therapy, total IGF-I concentrations were comparable in both groups (baseline: G+MET 13.3 ± 1.0 vs NPH+MET 13.3 ± 1.0 nmol/l, p = 0.97; and 36 weeks: 13.4 ± 1.0 vs 13.1 ± 0.9 nmol/l, p = 0.71). Total IGF-I concentration did not change during insulin therapy (13.3 ± 0.7 vs 13.3 ± 0.7 nmol/l, baseline vs 36 weeks, p = 0.86).
Conclusions/interpretation: Addition of insulin glargine or NPH insulin to metformin monotherapy in poorly controlled type 2 diabetic patients decreases serum IGF-I bioactivity in a similar manner.
Figures
References
-
- Food and Drug Administration (2011) FDA Drug Safety Communication: update to ongoing safety review of Lantus (insulin glargine) and possible risk of cancer. Available from www.fda.gov/Drugs/DrugSafety/ucm239376.htm, accessed 12 January 2011.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

