Mechanisms of p53 activation and physiological relevance in the developing kidney
- PMID: 22237799
- PMCID: PMC3330719
- DOI: 10.1152/ajprenal.00642.2011
Mechanisms of p53 activation and physiological relevance in the developing kidney
Abstract
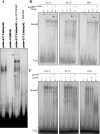
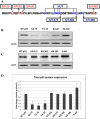
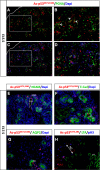
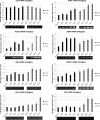
The tumor suppressor protein p53 is a short-lived transcription factor due to Mdm2-mediated proteosomal degradation. In response to genotoxic stress, p53 is stabilized via posttranslational modifications which prevent Mdm2 binding. p53 activation results in cell cycle arrest and apoptosis. We previously reported that tight regulation of p53 activity is an absolute requirement for normal nephron differentiation (Hilliard S, Aboudehen K, Yao X, El-Dahr SS Dev Biol 353: 354-366, 2011). However, the mechanisms of p53 activation in the developing kidney are unknown. We show here that metanephric p53 is phosphorylated and acetylated on key serine and lysine residues, respectively, in a temporal profile which correlates with the maturational changes in total p53 levels and DNA-binding activity. Site-directed mutagenesis revealed a differential role for these posttranslational modifications in mediating p53 stability and transcriptional regulation of renal function genes (RFGs). Section immunofluorescence also revealed that p53 modifications confer the protein with specific spatiotemporal expression patterns. For example, phos-p53(S392) is enriched in maturing proximal tubular epithelial cells, whereas acetyl-p53(K373/K382/K386) are expressed in nephron progenitors. Functionally, p53 occupancy of RFG promoters is enhanced at the onset of tubular differentiation, and p53 loss or gain of function indicates that p53 is necessary but not sufficient for RFG expression. We conclude that posttranslational modifications are important determinants of p53 stability and physiological functions in the developing kidney. We speculate that the stress/hypoxia of the embryonic microenvironment may provide the stimulus for p53 activation in the developing kidney.
Figures






References
-
- Amariglio F, Tchang F, Prioleau MN, Soussi T, Cibert C, Mechali M. A functional analysis of p53 during early development of Xenopus laevis. Oncogene 15: 2191– 2199, 1997 - PubMed
-
- Armstrong JF, Kaufman MH, Harrison DJ, Clarke AR. High-frequency developmental abnormalities in p53-deficient mice. Curr Biol 5: 931– 936, 1995 - PubMed
-
- Attardi LD, DePinho RA. Conquering the complexity of p53. Nat Genet 36: 7– 8, 2004 - PubMed
-
- Barlev NA, Liu L, Chehab NH, Mansfield K, Harris KG, Halazonetis TD, Berger SL. Acetylation of p53 activates transcription through recruitment of coactivators/histone acetyltransferases. Mol Cell 8: 1243– 1254, 2001 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

