Inner hair cells are not required for survival of spiral ganglion neurons in the adult cochlea
- PMID: 22238076
- PMCID: PMC3678770
- DOI: 10.1523/JNEUROSCI.4678-11.2012
Inner hair cells are not required for survival of spiral ganglion neurons in the adult cochlea
Erratum in
- J Neurosci. 2012 Apr 4;32(14):5016
Abstract
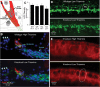
Studies of sensorineural hearing loss have long suggested that survival of spiral ganglion neurons (SGNs) depends on trophic support provided by their peripheral targets, the inner hair cells (IHCs): following ototoxic drugs or acoustic overexposure, IHC death is rapid whereas SGN degeneration is always delayed. However, recent noise-trauma studies show that SGNs can die even when hair cells survive, and transgenic mouse models show that supporting cell dysfunction can cause SGN degeneration in the absence of IHC pathology. To reexamine this issue, we studied a model of IHC loss that does not involve noise or ototoxic drugs. Mice lacking the gene for the high-affinity thiamine transporter (Slc19a2) have normal cochlear structure and function when fed a regular (thiamine-rich) diet. However, dietary thiamine restriction causes widespread, rapid (within 10 d) loss of IHCs. Using this model, we show that SGNs can survive for months after IHC loss, indicating that (1) IHCs are not necessary for neuronal survival, (2) neuronal loss in the other hearing loss models is likely due to effects of the trauma on the sensory neurons or other inner ear cells, and (3) that other cells, most likely supporting cells of the organ of Corti, are the main source of SGN survival factors. These results overturn a long-standing dogma in the study of sensorineural hearing loss and highlight the importance of cochlear supporting cells in neuronal survival in the adult inner ear.
Figures

References
-
- Bohne BA, Harding GW. Degeneration in the cochlea after noise damage: primary versus secondary events. Am J Otol. 2000;21:505–509. - PubMed
-
- Brown MC. Morphology of labeled efferent fibers in the guinea pig cochlea. J Comp Neurol. 1987;260:605–618. - PubMed
-
- Dupont J, Guilhaume A, Aran JM. Neuronal degeneration of primary cochlear and vestibular innervations after local injection of sisomicin in the guinea pig. Hear Res. 1993;68:217–228. - PubMed
-
- Fariñas I, Jones KR, Backus C, Wang XY, Reichardt LF. Severe sensory and sympathetic deficits in mice lacking neurotrophin-3. Nature. 1994;369:658–661. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
