Prenatal immune activation interacts with genetic Nurr1 deficiency in the development of attentional impairments
- PMID: 22238080
- PMCID: PMC6621065
- DOI: 10.1523/JNEUROSCI.4831-11.2012
Prenatal immune activation interacts with genetic Nurr1 deficiency in the development of attentional impairments
Abstract
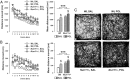
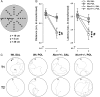
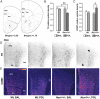
Prenatal exposure to infection has been linked to increased risk of neurodevelopmental brain disorders, and recent evidence implicates altered dopaminergic development in this association. However, since the relative risk size of prenatal infection appears relatively small with respect to long-term neuropsychiatric outcomes, it is likely that this prenatal insult interacts with other factors in shaping the risk of postnatal brain dysfunctions. In the present study, we show that the neuropathological consequences of prenatal viral-like immune activation are exacerbated in offspring with genetic predisposition to dopaminergic abnormalities induced by mutations in Nurr1, a transcription factor highly essential for normal dopaminergic development. We combined a mouse model of heterozygous genetic deletion of Nurr1 with a model of prenatal immune challenge by the viral mimetic poly(I:C) (polyriboinosinic polyribocytidilic acid). In our gene-environment interaction model, we demonstrate that the combination of the genetic and environmental factors not only exerts additive effects on locomotor hyperactivity and sensorimotor gating deficits, but further produces synergistic effects in the development of impaired attentional shifting and sustained attention. We further demonstrate that the combination of the two factors is necessary to trigger maldevelopment of prefrontal cortical and ventral striatal dopamine systems. Our findings provide evidence for specific gene-environment interactions in the emergence of enduring attentional impairments and neuronal abnormalities pertinent to dopamine-associated brain disorders such as schizophrenia and attention deficit/hyperactivity disorder, and further emphasize a critical role of abnormal dopaminergic development in these etiopathological processes.
Figures





References
-
- Abazyan B, Nomura J, Kannan G, Ishizuka K, Tamashiro KL, Nucifora F, Pogorelov V, Ladenheim B, Yang C, Krasnova IN, Cadet JL, Pardo C, Mori S, Kamiya A, Vogel MW, Sawa A, Ross CA, Pletnikov MV. Prenatal interaction of mutant DISC1 and immune activation produces adult psychopathology. Biol Psychiatry. 2010;68:1172–1181. - PMC - PubMed
-
- Akil M, Pierri JN, Whitehead RE, Edgar CL, Mohila C, Sampson AR, Lewis DA. Lamina-specific alterations in the dopamine innervation of the prefrontal cortex in schizophrenic subjects. Am J Psychiatry. 1999;156:1580–1589. - PubMed
-
- Baddeley A. Working memory: looking back and looking forward. Nat Rev Neurosci. 2003;4:829–839. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
