Adaptation of binaural processing in the adult brainstem induced by ambient noise
- PMID: 22238082
- PMCID: PMC6621091
- DOI: 10.1523/JNEUROSCI.2094-11.2012
Adaptation of binaural processing in the adult brainstem induced by ambient noise
Abstract
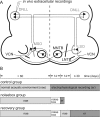
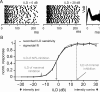
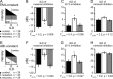
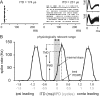
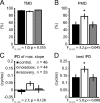
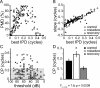
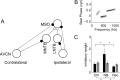
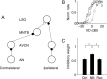
Interaural differences in stimulus intensity and timing are major cues for sound localization. In mammals, these cues are first processed in the lateral and medial superior olive by interaction of excitatory and inhibitory synaptic inputs from ipsi- and contralateral cochlear nucleus neurons. To preserve sound localization acuity following changes in the acoustic environment, the processing of these binaural cues needs neuronal adaptation. Recent studies have shown that binaural sensitivity adapts to stimulation history within milliseconds, but the actual extent of binaural adaptation is unknown. In the current study, we investigated long-term effects on binaural sensitivity using extracellular in vivo recordings from single neurons in the dorsal nucleus of the lateral lemniscus that inherit their binaural properties directly from the lateral and medial superior olives. In contrast to most previous studies, we used a noninvasive approach to influence this processing. Adult gerbils were exposed for 2 weeks to moderate noise with no stable binaural cue. We found monaural response properties to be unaffected by this measure. However, neuronal sensitivity to binaural cues was reversibly altered for a few days. Computational models of sensitivity to interaural time and level differences suggest that upregulation of inhibition in the superior olivary complex can explain the electrophysiological data.
Figures

Similar articles
-
Ambient noise exposure induces long-term adaptations in adult brainstem neurons.Sci Rep. 2021 Mar 4;11(1):5139. doi: 10.1038/s41598-021-84230-9. Sci Rep. 2021. PMID: 33664302 Free PMC article.
-
Robustness of neuronal tuning to binaural sound localization cues against age-related loss of inhibitory synaptic inputs.PLoS Comput Biol. 2021 Jul 9;17(7):e1009130. doi: 10.1371/journal.pcbi.1009130. eCollection 2021 Jul. PLoS Comput Biol. 2021. PMID: 34242210 Free PMC article.
-
Computational principles of neural adaptation for binaural signal integration.PLoS Comput Biol. 2020 Jul 17;16(7):e1008020. doi: 10.1371/journal.pcbi.1008020. eCollection 2020 Jul. PLoS Comput Biol. 2020. PMID: 32678847 Free PMC article.
-
Monaural and binaural processing in the ventral nucleus of the lateral lemniscus: a major source of inhibition to the inferior colliculus.Hear Res. 2002 Jun;168(1-2):90-7. doi: 10.1016/s0378-5955(02)00368-4. Hear Res. 2002. PMID: 12117512 Review.
-
Roles of inhibition for transforming binaural properties in the brainstem auditory system.Hear Res. 2002 Jun;168(1-2):60-78. doi: 10.1016/s0378-5955(02)00362-3. Hear Res. 2002. PMID: 12117510 Review.
Cited by
-
Congenital and prolonged adult-onset deafness cause distinct degradations in neural ITD coding with bilateral cochlear implants.J Assoc Res Otolaryngol. 2013 Jun;14(3):393-411. doi: 10.1007/s10162-013-0380-5. Epub 2013 Mar 5. J Assoc Res Otolaryngol. 2013. PMID: 23462803 Free PMC article.
-
Ambient noise exposure induces long-term adaptations in adult brainstem neurons.Sci Rep. 2021 Mar 4;11(1):5139. doi: 10.1038/s41598-021-84230-9. Sci Rep. 2021. PMID: 33664302 Free PMC article.
-
Regulation of conduction time along axons.Neuroscience. 2014 Sep 12;276:126-34. doi: 10.1016/j.neuroscience.2013.06.047. Epub 2013 Jun 29. Neuroscience. 2014. PMID: 23820043 Free PMC article. Review.
-
Adaptation in sound localization: from GABA(B) receptor-mediated synaptic modulation to perception.Nat Neurosci. 2013 Dec;16(12):1840-7. doi: 10.1038/nn.3548. Epub 2013 Oct 20. Nat Neurosci. 2013. PMID: 24141311
-
Glycinergic inhibition tunes coincidence detection in the auditory brainstem.Nat Commun. 2014 May 7;5:3790. doi: 10.1038/ncomms4790. Nat Commun. 2014. PMID: 24804642 Free PMC article.
References
-
- Alvarado JC, Fuentes-Santamaria V, Henkel CK, Brunso-Bechtold JK. Alterations in calretinin immunostaining in the ferret superior olivary complex after cochlear ablation. J Comp Neurol. 2004;470:63–79. - PubMed
-
- Batra R, Kuwada S, Fitzpatrick DC. Sensitivity to interaural temporal disparities of low- and high-frequency neurons in the superior olivary complex. I. Heterogeneity of responses. J Neurophysiol. 1997;78:1222–1236. - PubMed
-
- Blauert J. Spatial hearing: the psychophysics of human sound localization. Cambridge, MA: MIT; 1997.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
