L-DOPA impairs proteasome activity in parkinsonism through D1 dopamine receptor
- PMID: 22238104
- PMCID: PMC6621078
- DOI: 10.1523/JNEUROSCI.1541-11.2012
L-DOPA impairs proteasome activity in parkinsonism through D1 dopamine receptor
Abstract
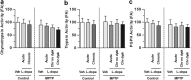
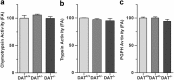
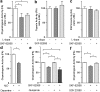
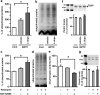
Aberrant membrane localization of dopamine D(1) receptor (D1R) is associated with L-DOPA-induced dyskinesia (LID), a major complication of L-DOPA treatment in Parkinson's disease (PD). Since the proteasome plays a central role in modulating neuronal response through regulation of neurotransmitter receptor intraneuronal fate, we hypothesized that the ubiquitine-proteasome proteolytic pathway could be impaired in LID. Those LIDs are actually associated with a striatum-specific decrease in proteasome catalytic activity and accumulation of polyubiquitinated proteins in experimental rodent and monkey parkinsonism. We then demonstrated that such decreased proteasome catalytic activity (1) results from D1R activation and (2) feed-back the D1R abnormal trafficking, i.e., its exaggerated cell surface abundance. We further showed that the genetic invalidation of the E3 ubiquitin-protein ligase parkin PD gene leads to exaggerated abnormal involuntary movements compared with wild-type mice. We thus established in an unprecedented series of experimental models that impairment of the ubiquitine-proteasome system at specific nodes (E3 ligase parkin, polyubiquitination, proteasome catalytic activity) leads to the same phenomenon, i.e., aberrant behavioral response to dopamine replacement therapy in PD, highlighting the intimate interplay between dopamine receptor and proteasome activity in a nondegenerative context.
Figures




Comment in
-
L-dopa impairs proteasome activity in Parkinsonism through D1 dopamine receptor.Mov Disord. 2012 May;27(6):706. doi: 10.1002/mds.24961. Mov Disord. 2012. PMID: 22822491 No abstract available.
References
-
- Ahmed MR, Berthet A, Bychkov E, Porras G, Li Q, Bioulac BH, Carl YT, Bloch B, Kook S, Aubert I, Dovero S, Doudnikoff E, Gurevich VV, Gurevich EV, Bezard E. Lentiviral overexpression of GRK6 alleviates L-dopa-induced dyskinesia in experimental Parkinson's disease. Sci Transl Med. 2010;2:28ra28. - PMC - PubMed
-
- Aubert I, Guigoni C, Håkansson K, Li Q, Dovero S, Barthe N, Bioulac BH, Gross CE, Fisone G, Bloch B, Bezard E. Increased D1 dopamine receptor signaling in levodopa-induced dyskinesia. Ann Neurol. 2005;57:17–26. - PubMed
-
- Benoit-Marand M, Jaber M, Gonon F. Release and elimination of dopamine in vivo in mice lacking the dopamine transporter: functional consequences. Eur J Neurosci. 2000;12:2985–2992. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
