The cycling of acetyl-coenzyme A through acetylcarnitine buffers cardiac substrate supply: a hyperpolarized 13C magnetic resonance study
- PMID: 22238215
- PMCID: PMC3378498
- DOI: 10.1161/CIRCIMAGING.111.969451
The cycling of acetyl-coenzyme A through acetylcarnitine buffers cardiac substrate supply: a hyperpolarized 13C magnetic resonance study
Abstract
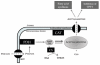
Background: Carnitine acetyltransferase catalyzes the reversible conversion of acetyl-coenzyme A (CoA) into acetylcarnitine. The aim of this study was to use the metabolic tracer hyperpolarized [2-(13)C]pyruvate with magnetic resonance spectroscopy to determine whether carnitine acetyltransferase facilitates carbohydrate oxidation in the heart.
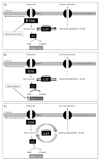
Methods and results: Ex vivo, following hyperpolarized [2-(13)C]pyruvate infusion, the [1-(13)C]acetylcarnitine resonance was saturated with a radiofrequency pulse, and the effect of this saturation on [1-(13)C]citrate and [5-(13)C]glutamate was observed. In vivo, [2-(13)C]pyruvate was infused into 3 groups of fed male Wistar rats: (1) controls, (2) rats in which dichloroacetate enhanced pyruvate dehydrogenase flux, and (3) rats in which dobutamine elevated cardiac workload. In the perfused heart, [1-(13)C]acetylcarnitine saturation reduced the [1-(13)C]citrate and [5-(13)C]glutamate resonances by 63% and 51%, respectively, indicating a rapid exchange between pyruvate-derived acetyl-CoA and the acetylcarnitine pool. In vivo, dichloroacetate increased the rate of [1-(13)C]acetylcarnitine production by 35% and increased the overall acetylcarnitine pool size by 33%. Dobutamine decreased the rate of [1-(13)C]acetylcarnitine production by 37% and decreased the acetylcarnitine pool size by 40%.
Conclusions: Hyperpolarized (13)C magnetic resonance spectroscopy has revealed that acetylcarnitine provides a route of disposal for excess acetyl-CoA and a means to replenish acetyl-CoA when cardiac workload is increased. Cycling of acetyl-CoA through acetylcarnitine appears key to matching instantaneous acetyl-CoA supply with metabolic demand, thereby helping to balance myocardial substrate supply and contractile function.
Figures




Comment in
-
In vivo clinical measures of intermediary metabolism are inadequate: can a new magnetic resonance spectroscopy technology do better?Circ Cardiovasc Imaging. 2012 Mar;5(2):171-4. doi: 10.1161/CIRCIMAGING.111.972240. Circ Cardiovasc Imaging. 2012. PMID: 22438422 No abstract available.
References
-
- Hansford RG, Cohen L. Relative importance of pyruvate dehydrogenase interconversion and feed-back inhibition in the effect of fatty acids on pyruvate oxidation by rat heart mitochondria. Arch Biochem Biophys. 1978;191:65–81. - PubMed
-
- Collins-Nakai RL, Noseworthy D, Lopaschuk GD. Epinephrine increases atp production in hearts by preferentially increasing glucose metabolism. The American journal of physiology. 1994;267:H1862–1871. - PubMed
-
- Owen OE, Mozzoli MA, Boden G, Patel MS, Reichard GA, Jr., Trapp V, Shuman CR, Felig P. Substrate, hormone, and temperature responses in males and females to a common breakfast. Metabolism. 1980;29:511–523. - PubMed
-
- Cross HR, Opie LH, Radda GK, Clarke K. Is a high glycogen content beneficial or detrimental to the ischemic rat heart? A controversy resolved. Circulation research. 1996;78:482–491. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

