Toxicity and efficacy of the acetylcholinesterase (AChe) inhibitor donepezil in childhood brain tumor survivors: a pilot study
- PMID: 22238217
- PMCID: PMC3345166
- DOI: 10.1002/pbc.24078
Toxicity and efficacy of the acetylcholinesterase (AChe) inhibitor donepezil in childhood brain tumor survivors: a pilot study
Abstract
Background: Neurocognitive deficits are a recognized late effect of curative brain tumor therapy. We evaluated the feasibility, tolerance, and impact of a pilot pharmacologic intervention with the acetylcholinesterase (AChe) inhibitor, donepezil, in pediatric brain tumor (BT) survivors at risk for neurocognitive dysfunction.
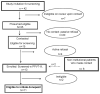
Procedure: A single institution open-label pilot study was conducted in childhood BT survivors: ≥1 year from cancer treatment; and who received >23.5 Gy cranial radiation therapy (RT). Toxicity, adherence and neurocognitive outcomes were evaluated at baseline and serially during 24 weeks of donepezil, and following a 12-week washout period off drug.
Results: From a pool of subjects, 13 were successfully contacted and screened, and 11 met all eligibility criteria to initiate donepezil at a median of 4.7 (1.9-11.9) years from RT. Seventy-two percent of patients completed the 24-week drug study visit. Despite transient gastrointestinal toxicity (vomiting and diarrhea) in 30% of patients there was no weight loss on donepezil. Significant improvement in performance was noted at 24 weeks on the Dellis-Kaplan Executive Function (D-KEF) Tower test (P < 0.001), the Wide Range Assessment of Memory and Learning, 2nd Edition (WRAML-2) Visual memory (P = 0.007), and the Number/Letter task (P = 0.018).
Conclusions: Donepezil was well tolerated among childhood BT survivors who had received substantial prior therapy. Based on improved executive function and memory performance in this pilot trial, a randomized placebo controlled trial of this pharmacologic agent is warranted to fully evaluate its efficacy in remediating neurocognitive dysfunction.
Copyright © 2012 Wiley Periodicals, Inc.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures
Similar articles
-
A study of donepezil in female breast cancer survivors with self-reported cognitive dysfunction 1 to 5 years following adjuvant chemotherapy.J Cancer Surviv. 2016 Feb;10(1):176-84. doi: 10.1007/s11764-015-0463-x. Epub 2015 Jul 1. J Cancer Surviv. 2016. PMID: 26130292 Free PMC article. Clinical Trial.
-
Cognitive effects of donepezil therapy in patients with brain tumors: a pilot study.J Neurooncol. 2016 Apr;127(2):313-9. doi: 10.1007/s11060-015-2035-3. Epub 2016 Jan 6. J Neurooncol. 2016. PMID: 26738844 Free PMC article.
-
An open-label pilot study of acetylcholinesterase inhibitors to promote functional recovery in elderly cognitively impaired stroke patients.Cerebrovasc Dis. 2008;26(3):317-21. doi: 10.1159/000149580. Epub 2008 Jul 31. Cerebrovasc Dis. 2008. PMID: 18667813 Free PMC article. Clinical Trial.
-
Perspectives in the management of Alzheimer's disease: clinical profile of donepezil.Dement Geriatr Cogn Disord. 1998;9 Suppl 3:29-42. doi: 10.1159/000051201. Dement Geriatr Cogn Disord. 1998. PMID: 9853200 Review.
-
Clinical benefits of a new piperidine-class AChE inhibitor.Eur Neuropsychopharmacol. 1999 Apr;9 Suppl 2:S69-77. doi: 10.1016/s0924-977x(98)00047-9. Eur Neuropsychopharmacol. 1999. PMID: 10332937 Review.
Cited by
-
Neurocognitive and Psychosocial Outcomes in Pediatric Brain Tumor Survivors.Bioengineering (Basel). 2018 Sep 11;5(3):73. doi: 10.3390/bioengineering5030073. Bioengineering (Basel). 2018. PMID: 30208602 Free PMC article. Review.
-
The Cholinesterase Inhibitory Properties of Stephaniae Tetrandrae Radix.Molecules. 2020 Dec 14;25(24):5914. doi: 10.3390/molecules25245914. Molecules. 2020. PMID: 33327436 Free PMC article.
-
Changes in Imaging and Cognition in Juvenile Rats After Whole-Brain Irradiation.Int J Radiat Oncol Biol Phys. 2016 Oct 1;96(2):470-478. doi: 10.1016/j.ijrobp.2016.06.013. Epub 2016 Jun 16. Int J Radiat Oncol Biol Phys. 2016. PMID: 27478168 Free PMC article.
-
Neurocognitive Outcomes and Interventions in Long-Term Survivors of Childhood Cancer.J Clin Oncol. 2018 Jul 20;36(21):2181-2189. doi: 10.1200/JCO.2017.76.4696. Epub 2018 Jun 6. J Clin Oncol. 2018. PMID: 29874137 Free PMC article. Review.
-
Working memory training in survivors of pediatric cancer: a randomized pilot study.Psychooncology. 2013 Aug;22(8):1856-65. doi: 10.1002/pon.3222. Epub 2012 Dec 2. Psychooncology. 2013. PMID: 23203754 Free PMC article. Clinical Trial.
References
-
- Mariotto AB, Rowland JH, Yabroff KR, et al. Long-term survivors of childhood cancers in the united states. Cancer Epidemiol Biomarkers Prev. 2009;18:1033–1040. - PubMed
-
- Nathan PC, Patel SK, Dilley K, et al. Guidelines for identification of, advocacy for, and intervention in neurocognitive problems in survivors of childhood cancer: A report from the children’s oncology group. Archives of pediatrics & adolescent medicine. 2007;161:798–806. - PubMed
-
- Mulhern RK, Merchant TE, Gajjar A, et al. Late neurocognitive sequelae in survivors of brain tumours in childhood. Lancet Oncol. 2004;5:399–408. - PubMed
-
- Bhat SR, Goodwin TL, Burwinkle TM, et al. Profile of daily life in children with brain tumors: An assessment of health-related quality of life. J Clin Oncol. 2005;23:5493–5500. - PubMed
-
- Zebrack BJ, Gurney JG, Oeffinger K, et al. Psychological outcomes in long-term survivors of childhood brain cancer: A report from the childhood cancer survivor study. J Clin Oncol. 2004;22:999–1006. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous