Varicella-zoster virus infects human embryonic stem cell-derived neurons and neurospheres but not pluripotent embryonic stem cells or early progenitors
- PMID: 22238301
- PMCID: PMC3302301
- DOI: 10.1128/JVI.06810-11
Varicella-zoster virus infects human embryonic stem cell-derived neurons and neurospheres but not pluripotent embryonic stem cells or early progenitors
Abstract
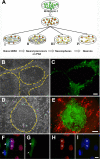
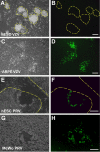
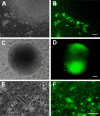
Pluripotent human stem cells are a powerful tool for the generation of differentiated cells that can be used for the study of human disease. We recently demonstrated that neurons derived from pluripotent human embryonic stem cells (hESC) can be infected by the highly host-restricted human alphaherpesvirus varicella-zoster virus (VZV), permitting the interaction of VZV with neurons to be readily evaluated in culture. In the present study, we examine whether pluripotent hESC and neural progenitors at intermediate stages of differentiation are permissive for VZV infection. We demonstrate here that VZV infection is blocked in naïve hESC. A block to VZV replication is also seen when a bacterial artificial chromosome (BAC) containing the VZV genome is transfected into hESC. In contrast, related alphaherpesviruses herpes simplex virus 1 (HSV-1) and pseudorabies virus (PrV) productively infect naïve hESC in a cell-free manner, and PrV replicates from a BAC transfected into hESC. Neurons differentiate from hESC via neural progenitor intermediates, as is the case in the embryo. The first in vitro stage at which permissiveness of hESC-derived neural precursors to VZV replication is observed is upon formation of "neurospheres," immediately after detachment from the inductive stromal feeder layer. These findings suggest that hESC may be useful in deciphering the yet enigmatic mechanisms of specificity of VZV infection and replication.
Figures

References
-
- Brokhman I, et al. 2009. Genetic modification of human embryonic stem cells with adenoviral vectors: differences of infectability between lines and correlation of infectability with expression of the coxsackie and adenovirus receptor. Stem Cells Dev. 18:447–456 - PubMed
-
- Cowan CA, et al. 2004. Derivation of embryonic stem-cell lines from human blastocysts. N. Engl. J. Med. 350:1353–1356 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

