Restricted replication of xenotropic murine leukemia virus-related virus in pigtailed macaques
- PMID: 22238316
- PMCID: PMC3302341
- DOI: 10.1128/JVI.06886-11
Restricted replication of xenotropic murine leukemia virus-related virus in pigtailed macaques
Abstract
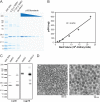
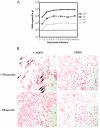
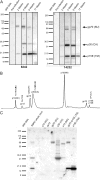
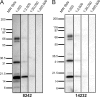
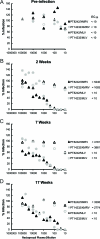
Although xenotropic murine leukemia virus-related virus (XMRV) has been previously linked to prostate cancer and myalgic encephalomyelitis/chronic fatigue syndrome, recent data indicate that results interpreted as evidence of human XMRV infection reflect laboratory contamination rather than authentic in vivo infection. Nevertheless, XMRV is a retrovirus of undefined pathogenic potential that is able to replicate in human cells. Here we describe a comprehensive analysis of two male pigtailed macaques (Macaca nemestrina) experimentally infected with XMRV. Following intravenous inoculation with >10(10) RNA copy equivalents of XMRV, viral replication was limited and transient, peaking at ≤2,200 viral RNA (vRNA) copies/ml plasma and becoming undetectable by 4 weeks postinfection, though viral DNA (vDNA) in peripheral blood mononuclear cells remained detectable through 119 days of follow-up. Similarly, vRNA was not detectable in lymph nodes by in situ hybridization despite detectable vDNA. Sequencing of cell-associated vDNA revealed extensive G-to-A hypermutation, suggestive of APOBEC-mediated viral restriction. Consistent with limited viral replication, we found transient upregulation of type I interferon responses that returned to baseline by 2 weeks postinfection, no detectable cellular immune responses, and limited or no spread to prostate tissue. Antibody responses, including neutralizing antibodies, however, were detectable by 2 weeks postinfection and maintained throughout the study. Both animals were healthy for the duration of follow-up. These findings indicate that XMRV replication and spread were limited in pigtailed macaques, predominantly by APOBEC-mediated hypermutation. Given that human APOBEC proteins restrict XMRV infection in vitro, human XMRV infection, if it occurred, would be expected to be characterized by similarly limited viral replication and spread.
Figures




References
-
- Akgul B, et al. 6 September 2011. No evidence for a role of xenotropic murine leukaemia virus-related virus and BK virus in prostate cancer of German patients. Med. Microbiol. Immunol. [Epub ahead of print.] doi:10.1007/s00430-011-0215-0 - DOI - PubMed
-
- Arnold RS, et al. 2010. XMRV infection in patients with prostate cancer: novel serologic assay and correlation with PCR and FISH. Urology 75:755–761 - PubMed
-
- Balada E, Castro-Marrero J, Felip L, Vilardell-Tarres M, Ordi-Ros J. 2011. Xenotropic murine leukemia virus-related virus (XMRV) in patients with systemic lupus erythematosus. J. Clin. Immunol. 31:584–587 - PubMed
-
- Barnes E, et al. 2010. Failure to detect xenotropic murine leukemia virus-related virus in blood of individuals at high risk of blood-borne viral infections. J. Infect. Dis. 202:1482–1485 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

