Magnetic resonance of 2-hydroxyglutarate in IDH1-mutated low-grade gliomas
- PMID: 22238333
- PMCID: PMC3772177
- DOI: 10.1126/scitranslmed.3002796
Magnetic resonance of 2-hydroxyglutarate in IDH1-mutated low-grade gliomas
Abstract
Recent studies have indicated that a significant survival advantage is conferred to patients with gliomas whose lesions harbor mutations in the genes isocitrate dehydrogenase 1 and 2 (IDH1/2). IDH1/2 mutations result in aberrant enzymatic production of the potential oncometabolite D-2-hydroxyglutarate (2HG). Here, we report on the ex vivo detection of 2HG in IDH1-mutated tissue samples from patients with recurrent low-grade gliomas using the nuclear magnetic resonance technique of proton high-resolution magic angle spinning spectroscopy. Relative 2HG levels from pathologically confirmed mutant IDH1 tissues correlated with levels of other ex vivo metabolites and histopathology parameters associated with increases in mitotic activity, relative tumor content, and cellularity. Ex vivo spectroscopic measurements of choline-containing species and in vivo magnetic resonance measurements of diffusion parameters were also correlated with 2HG levels. These data provide extensive characterization of mutant IDH1 lesions while confirming the potential diagnostic value of 2HG as a surrogate marker of patient survival. Such information may augment the ability of clinicians to monitor therapeutic response and provide criteria for stratifying patients to specific treatment regimens.
Figures
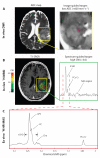



References
-
- Grier JT, Batchelor T. Low-grade gliomas in adults. Oncologist. 2006;11:681–693. - PubMed
-
- Riemenschneider MJ, Reifenberger G. Molecular neuropathology of low-grade gliomas and its clinical impact. Adv. Tech. Stand. Neurosurg. 2010;35:35–64. - PubMed
-
- Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Siu IM, Gallia GL, Olivi A, McLendon R, Rasheed BA, Keir S, Nikolskaya T, Nikolsky Y, Busam DA, Tekleab H, Diaz LA, Jr., Hartigan J, Smith DR, Strausberg RL, Marie SK, Shinjo SM, Yan H, Riggins GJ, Bigner DD, Karchin R, Papadopoulos N, Parmigiani G, Vogelstein B, Velculescu VE, Kinzler KW. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. - PMC - PubMed
-
- Houillier C, Wang X, Kaloshi G, Mokhtari K, Guillevin R, Laffaire J, Paris S, Boisselier B, Idbaih A, Laigle-Donadey F, Hoang-Xuan K, Sanson M, Delattre JY. IDH1 or IDH2 mutations predict longer survival and response to temozolomide in low-grade gliomas. Neurology. 2010;75:1560–1566. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

