Update on bone anabolics in osteoporosis treatment: rationale, current status, and perspectives
- PMID: 22238383
- PMCID: PMC3275361
- DOI: 10.1210/jc.2011-2332
Update on bone anabolics in osteoporosis treatment: rationale, current status, and perspectives
Abstract
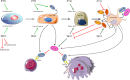
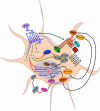
Osteoporosis is defined as low bone mineral density associated with skeletal fractures secondary to minimal or no trauma, most often involving the spine, the hip, and the forearm. The decrease in bone mineral density is the consequence of an unbalanced bone remodeling process, with higher bone resorption than bone formation. Osteoporosis affects predominantly postmenopausal women, but also older men. This chronic disease represents a considerable medical and socioeconomic burden for modern societies. The therapeutic options for the treatment of osteoporosis have so far comprised mostly antiresorptive drugs, in particular bisphosphonates and more recently denosumab, but also calcitonin and, for women, estrogens or selective estrogen receptor modulators. These drugs have limitations, however, in particular the fact that they lead to a low turnover state where bone formation decreases with the decrease in bone-remodeling activity. In this review, we discuss the alternative class of osteoporosis drugs, i.e. bone anabolics, their biology, and the perspectives they offer for our therapeutic armamentarium. We focus on the two main osteoanabolic pathways identified as of today: PTH, the only anabolic drug currently on the market; and activation of canonical Wnt signaling through inhibition of the endogenous inhibitors sclerostin and dickkopf1. Each approach is based on a different molecular mechanism, but most recent evidence suggests that these two pathways may actually converge, at least in part. Whereas recombinant human PTH treatment is being revisited with different formulations and attempts to regulate endogenous PTH secretion via the calcium-sensing receptor, antibodies to sclerostin and dickkopf1 are currently in clinical trials and may prove to be even more efficient at increasing bone mass, possibly independent of bone turnover. Each of these anabolic approaches has its own limitations and safety issues, but the prospects of effective anabolic therapy for osteoporosis are indeed bright.
Figures

References
-
- Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. 1987. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 2:595–610 - PubMed
-
- Harvey N, Dennison E, Cooper C. 2010. Osteoporosis: impact on health and economics. Nat Rev Rheumatol 6:99–105 - PubMed
-
- Hollick RJ, Reid DM. 2011. Role of bisphosphonates in the management of postmenopausal osteoporosis: an update on recent safety anxieties. Menopause Int 17:66–72 - PubMed
-
- Spiro AS, Beil FT, Timo Beil F, Schinke T, Schilling AF, Eulenburg C, Rueger JM, Amling M. 2010. Short-term application of dexamethasone enhances bone morphogenetic protein-7-induced ectopic bone formation in vivo. J Trauma 69:1473–1480 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

