TsHKT1;2, a HKT1 homolog from the extremophile Arabidopsis relative Thellungiella salsuginea, shows K(+) specificity in the presence of NaCl
- PMID: 22238420
- PMCID: PMC3291249
- DOI: 10.1104/pp.111.193110
TsHKT1;2, a HKT1 homolog from the extremophile Arabidopsis relative Thellungiella salsuginea, shows K(+) specificity in the presence of NaCl
Abstract
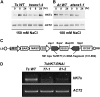
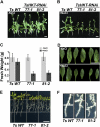
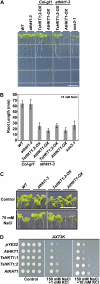
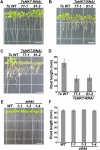
Cellular Na(+)/K(+) ratio is a crucial parameter determining plant salinity stress resistance. We tested the function of plasma membrane Na(+)/K(+) cotransporters in the High-affinity K(+) Transporter (HKT) family from the halophytic Arabidopsis (Arabidopsis thaliana) relative Thellungiella salsuginea. T. salsuginea contains at least two HKT genes. TsHKT1;1 is expressed at very low levels, while the abundant TsHKT1;2 is transcriptionally strongly up-regulated by salt stress. TsHKT-based RNA interference in T. salsuginea resulted in Na(+) sensitivity and K(+) deficiency. The athkt1 mutant lines overexpressing TsHKT1;2 proved less sensitive to Na(+) and showed less K(+) deficiency than lines overexpressing AtHKT1. TsHKT1;2 ectopically expressed in yeast mutants lacking Na(+) or K(+) transporters revealed strong K(+) transporter activity and selectivity for K(+) over Na(+). Altering two amino acid residues in TsHKT1;2 to mimic the AtHKT1 sequence resulted in enhanced sodium uptake and loss of the TsHKT1;2 intrinsic K(+) transporter activity. We consider the maintenance of K(+) uptake through TsHKT1;2 under salt stress an important component supporting the halophytic lifestyle of T. salsuginea.
Figures


Similar articles
-
Role of HKT1 in Thellungiella salsuginea, a model extremophile plant.Plant Signal Behav. 2013 Aug;8(8):e25196. doi: 10.4161/psb.25196. Epub 2013 Jun 10. Plant Signal Behav. 2013. PMID: 23759555 Free PMC article.
-
A Single Amino-Acid Substitution in the Sodium Transporter HKT1 Associated with Plant Salt Tolerance.Plant Physiol. 2016 Jul;171(3):2112-26. doi: 10.1104/pp.16.00569. Epub 2016 May 9. Plant Physiol. 2016. PMID: 27208305 Free PMC article.
-
The interaction complexes of TsHKT1 splicing variants enhance salt tolerance of Thellungiella salsuginea by decreasing Na+ uptake.Plant Sci. 2025 Oct;359:112678. doi: 10.1016/j.plantsci.2025.112678. Epub 2025 Jul 23. Plant Sci. 2025. PMID: 40712791
-
A conserved primary salt tolerance mechanism mediated by HKT transporters: a mechanism for sodium exclusion and maintenance of high K(+)/Na(+) ratio in leaves during salinity stress.Plant Cell Environ. 2010 Apr;33(4):552-65. doi: 10.1111/j.1365-3040.2009.02056.x. Epub 2009 Nov 4. Plant Cell Environ. 2010. PMID: 19895406 Review.
-
To exclude or to accumulate? Revealing the role of the sodium HKT1;5 transporter in plant adaptive responses to varying soil salinity.Plant Physiol Biochem. 2021 Dec;169:333-342. doi: 10.1016/j.plaphy.2021.11.030. Epub 2021 Nov 19. Plant Physiol Biochem. 2021. PMID: 34837866 Review.
Cited by
-
Physiological and molecular mechanisms of plant salt tolerance.Photosynth Res. 2013 May;115(1):1-22. doi: 10.1007/s11120-013-9813-6. Epub 2013 Mar 29. Photosynth Res. 2013. PMID: 23539361 Review.
-
Role of HKT1 in Thellungiella salsuginea, a model extremophile plant.Plant Signal Behav. 2013 Aug;8(8):e25196. doi: 10.4161/psb.25196. Epub 2013 Jun 10. Plant Signal Behav. 2013. PMID: 23759555 Free PMC article.
-
Advances in studies on ion transporters involved in salt tolerance and breeding crop cultivars with high salt tolerance.J Zhejiang Univ Sci B. 2020 Jun;21(6):426-441. doi: 10.1631/jzus.B1900510. J Zhejiang Univ Sci B. 2020. PMID: 32478490 Free PMC article. Review.
-
Halophytes: Potential Resources for Salt Stress Tolerance Genes and Promoters.Front Plant Sci. 2017 May 18;8:829. doi: 10.3389/fpls.2017.00829. eCollection 2017. Front Plant Sci. 2017. PMID: 28572812 Free PMC article. Review.
-
Structural insights into ion selectivity and transport mechanisms of Oryza sativa HKT2;1 and HKT2;2/1 transporters.Nat Plants. 2024 Apr;10(4):633-644. doi: 10.1038/s41477-024-01665-4. Epub 2024 Apr 3. Nat Plants. 2024. PMID: 38570642
References
-
- Adams P, Nelson DE, Yamada S, Chmara W, Jensen RG, Bohnert HJ, Griffiths H. (1998) Growth and development of Mesembryanthemum crystallinum. New Phytol 138: 171–190 - PubMed
-
- Apse MP, Aharon GS, Snedden WA, Blumwald E. (1999) Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis. Science 285: 1256–1258 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

