Comparison of commercial extraction systems and PCR assays for quantification of Epstein-Barr virus DNA load in whole blood
- PMID: 22238432
- PMCID: PMC3318550
- DOI: 10.1128/JCM.05593-11
Comparison of commercial extraction systems and PCR assays for quantification of Epstein-Barr virus DNA load in whole blood
Abstract
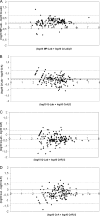
The automation of DNA extraction and the use of commercial quantitative real-time PCR assays could help obtain more reliable results for the quantification of Epstein-Barr virus DNA loads (EBV VL). This study compared two automated extraction platforms and two commercial PCRs for measurement of EBV VL in 10 EBV specimens from Quality Control for Molecular Diagnostics (QCMD) and in 200 whole-blood (WB) specimens from transplant (n = 137) and nontransplant (n = 63) patients. The WB specimens were extracted using the QIAcube or MagNA Pure instrument; VL were quantified with the EBV R-gene quantification kit (Argene) or the artus EBV RG PCR kit (Qiagen) on the Rotor-Gene 6000 real-time analyzer; and the results were compared with those of a laboratory-developed PCR. DNA was extracted from the QCMD specimens by use of the QIAamp DNA minikit and was quantified by the three PCR assays. The extraction platforms and the PCR assays showed good correlation (R, >0.9; P, <0.0001), but as many as 10% discordant results were observed, mostly for low viral loads (<3 log(10) copies/ml), and standard deviations reached as high as 0.49 log(10) copy/ml. In WB but not in QCMD samples, Argene PCR tended to give higher VL values than artus PCR or the laboratory-developed PCR (mean difference for the 200 WB VL, -0.42 or -0.36, respectively). In conclusion, the two automated extraction platforms and the two PCRs provided reliable and comparable VL results, but differences greater than 0.5 log(10) copy/ml remained between the two commercial PCRs after common DNA extraction.
Figures

References
-
- Abbate I, et al. 2011. Multicenter comparative study of Epstein-Barr virus DNA quantification for virological monitoring in transplanted patients. J. Clin. Virol. 50:224–229 - PubMed
-
- Ahsanuddin AN, Standish MC, Caliendo AM, Hill CE, Nolte FS. 2008. Validation of an Epstein-Barr viral load assay using the QIAGEN Artus EBV TM PCR analyte-specific reagent. Am. J. Clin. Pathol. 130:865–869 - PubMed
-
- Brengel-Pesce K, et al. 2002. Routine use of real-time quantitative PCR for laboratory diagnosis of Epstein-Barr virus infections. J. Med. Virol. 66:360–369 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

