Photochemical and thermal intramolecular 1,3-dipolar cycloaddition reactions of new o-stilbene-methylene-3-sydnones and their synthesis
- PMID: 22238545
- PMCID: PMC3252871
- DOI: 10.3762/bjoc.7.196
Photochemical and thermal intramolecular 1,3-dipolar cycloaddition reactions of new o-stilbene-methylene-3-sydnones and their synthesis
Abstract
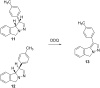
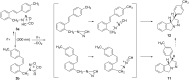
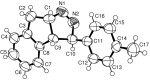
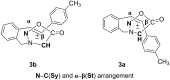
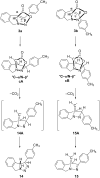
New trans- and cis-o-stilbene-methylene-sydnones 3a,b were synthesized by transforming the trans- and cis-o-aminomethylstilbene derivative, obtained by reduction of corresponding o-cyano derivatives, into glycine ester derivatives (43 and 31% yield) followed by hydrolysis (90 and 96% yield), nitrosation and ring closure with acetic acid anhydride (30 and 40% yield). The products were submitted to photochemical and thermal intramolecular [3 + 2] cycloadditions to afford diverse heteropolycyclic compounds. Photochemical reactions afforded cis-3-(4-methylphenyl)-3a,8-dihydro-3H-pyrazolo[5,1-a]isoindole (11, 12.5% yield) and trans-3-(4-methylphenyl)-3a,8-dihydro-3H-pyrazolo[5,1-a]isoindole (12, 5% yield). Thermal reactions afforded 3-(4-methylphenyl)-3,3a,8,8a-tetrahydroindeno[2,1-c]pyrazole (14, 50% yield) and 11-(4-methylphenyl)-9,10-diazatricyclo[7.2.1.0(2,7)]dodeca-2,4,6,10-tetraene (15, 22% yield).
Keywords: [3 + 2] cycloaddition; isoindoles; nitrile imines; pyrazoles; sydnones.
Figures


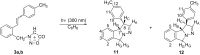
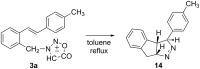
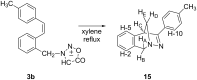

References
-
- Stewart F H C. Chem Rev. 1964;64:129–147. doi: 10.1021/cr60228a004. - DOI
-
- Newton C G, Ramsden C A. Tetrahedron. 1982;38:2965–3011. doi: 10.1016/0040-4020(82)80186-5. - DOI
-
- Clapp L B. 1,2,3- and 1,2,4-Oxadiazoles. In: Katritzky A R, Rees C W, editors. Comprehensive Heterocyclic Chemistry. Vol. 6. Oxford: Pergamon Press; 1984. pp. 365–378. - DOI
-
- Gribble G W. Mesoionic Ring Systems. In: Padwa A, Pearson W H, editors. Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry Toward Heterocycles and Natural Products. New Jersey: Wiley & Sons; 2003. pp. 681–753.
-
- Browne D L, Harrity J P A. Tetrahedron. 2010;66:553–568. doi: 10.1016/j.tet.2009.10.085. - DOI
LinkOut - more resources
Full Text Sources
