Trihydrophobin 1 phosphorylation by c-Src regulates MAPK/ERK signaling and cell migration
- PMID: 22238675
- PMCID: PMC3253115
- DOI: 10.1371/journal.pone.0029920
Trihydrophobin 1 phosphorylation by c-Src regulates MAPK/ERK signaling and cell migration
Abstract
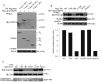
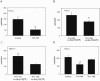
c-Src activates Ras-MAPK/ERK signaling pathway and regulates cell migration, while trihydrophobin 1 (TH1) inhibits MAPK/ERK activation and cell migration through interaction with A-Raf and PAK1 and inhibiting their kinase activities. Here we show that c-Src interacts with TH1 by GST-pull down assay, coimmunoprecipitation and confocal microscopy assay. The interaction leads to phosphorylation of TH1 at Tyr-6 in vivo and in vitro. Phosphorylation of TH1 decreases its association with A-Raf and PAK1. Further study reveals that Tyr-6 phosphorylation of TH1 reduces its inhibition on MAPK/ERK signaling, enhances c-Src mediated cell migration. Moreover, induced tyrosine phosphorylation of TH1 has been found by EGF and estrogen treatments. Taken together, our findings demonstrate a novel mechanism for the comprehensive regulation of Ras/Raf/MEK/ERK signaling and cell migration involving tyrosine phosphorylation of TH1 by c-Src.
Conflict of interest statement
Figures





Similar articles
-
Trihydrophobin 1 Interacts with PAK1 and Regulates ERK/MAPK Activation and Cell Migration.J Biol Chem. 2009 Mar 27;284(13):8786-96. doi: 10.1074/jbc.M806144200. Epub 2009 Jan 9. J Biol Chem. 2009. PMID: 19136554 Free PMC article.
-
Epidermal growth factor stimulates RSK2 activation through activation of the MEK/ERK pathway and src-dependent tyrosine phosphorylation of RSK2 at Tyr-529.J Biol Chem. 2008 Feb 22;283(8):4652-7. doi: 10.1074/jbc.M709673200. Epub 2007 Dec 21. J Biol Chem. 2008. PMID: 18156174 Free PMC article.
-
Role of c-Src tyrosine kinase in G protein-coupled receptor- and Gbetagamma subunit-mediated activation of mitogen-activated protein kinases.J Biol Chem. 1996 Aug 9;271(32):19443-50. doi: 10.1074/jbc.271.32.19443. J Biol Chem. 1996. PMID: 8702633
-
Rapid and transient activation of the ERK MAPK signalling pathway by macrophage migration inhibitory factor (MIF) and dependence on JAB1/CSN5 and Src kinase activity.Cell Signal. 2006 May;18(5):688-703. doi: 10.1016/j.cellsig.2005.06.013. Epub 2005 Aug 24. Cell Signal. 2006. PMID: 16122907
-
C-terminal Src kinase-mediated EPIYA phosphorylation of Pragmin creates a feed-forward C-terminal Src kinase activation loop that promotes cell motility.Cancer Sci. 2016 Jul;107(7):972-80. doi: 10.1111/cas.12962. Epub 2016 Jun 13. Cancer Sci. 2016. PMID: 27116701 Free PMC article.
Cited by
-
Role of LPS-elicited signaling in triggering gastric mucosal inflammatory responses to H. pylori: modulatory effect of ghrelin.Inflammopharmacology. 2017 Aug;25(4):415-429. doi: 10.1007/s10787-017-0360-1. Epub 2017 May 17. Inflammopharmacology. 2017. PMID: 28516374 Review.
-
Induction in gastric mucosal prostaglandin and nitric oxide by Helicobacter pylori is dependent on MAPK/ERK-mediated activation of IKK-β and cPLA2: modulatory effect of ghrelin.Inflammopharmacology. 2013 Jun;21(3):241-51. doi: 10.1007/s10787-013-0169-5. Epub 2013 Apr 6. Inflammopharmacology. 2013. PMID: 23563696
-
Sphingosine-1-phosphate suppresses chondrosarcoma metastasis by upregulation of tissue inhibitor of metalloproteinase 3 through suppressing miR-101 expression.Mol Oncol. 2017 Oct;11(10):1380-1398. doi: 10.1002/1878-0261.12106. Epub 2017 Aug 8. Mol Oncol. 2017. PMID: 28672103 Free PMC article.
-
Hispolon Methyl Ether, a Hispolon Analog, Suppresses the SRC/STAT3/Survivin Signaling Axis to Induce Cytotoxicity in Human Urinary Bladder Transitional Carcinoma Cell Lines.Int J Mol Sci. 2022 Dec 21;24(1):138. doi: 10.3390/ijms24010138. Int J Mol Sci. 2022. PMID: 36613579 Free PMC article.
-
Discovery of specific metastasis-related N-glycan alterations in epithelial ovarian cancer based on quantitative glycomics.PLoS One. 2014 Feb 6;9(2):e87978. doi: 10.1371/journal.pone.0087978. eCollection 2014. PLoS One. 2014. PMID: 24516574 Free PMC article.
References
-
- Yeatman TJ. A renaissance for SRC. Nat Rev Cancer. 2004;4:470–480. - PubMed
-
- Frame MC. Src in cancer: deregulation and consequences for cell behaviour. Biochim Biophys Acta. 2002;1602:114–130. - PubMed
-
- Soriano P, Montgomery C, Geske R, Bradley A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell. 1991;64:693–702. - PubMed
-
- Ishizawar R, Parsons SJ. c-Src and cooperating partners in human cancer. Cancer Cell. 2004;6:209–214. - PubMed
-
- Mayer EL, Krop IE. Advances in targeting SRC in the treatment of breast cancer and other solid malignancies. Clin Cancer Res. 2010;16:3526–3532. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

