Positive autoregulation of GDNF levels in the ventral tegmental area mediates long-lasting inhibition of excessive alcohol consumption
- PMID: 22238721
- PMCID: PMC3253655
- DOI: 10.1038/tp.2011.57
Positive autoregulation of GDNF levels in the ventral tegmental area mediates long-lasting inhibition of excessive alcohol consumption
Abstract
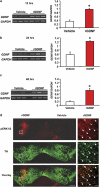
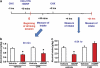
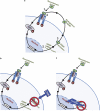
Glial cell line-derived neurotrophic factor (GDNF) is an essential growth factor for the survival and maintenance of the midbrain dopaminergic (DA-ergic) neurons. Activation of the GDNF pathway in the ventral tegmental area (VTA), where the GDNF receptors are expressed, produces a long-lasting suppression of excessive alcohol consumption in rats. Previous studies conducted in the DA-ergic-like cells, SHSY5Y, revealed that GDNF positively regulates its own expression, leading to a long-lasting activation of the GDNF signaling pathway. Here we determined whether GDNF activates a positive autoregulatory feedback loop in vivo within the VTA, and if so, whether this mechanism underlies the long-lasting suppressive effects of the growth factor on excessive alcohol consumption. We found that a single infusion of recombinant GDNF (rGDNF; 10 μg) into the VTA induces a long-lasting local increase in GDNF mRNA and protein levels, which depends upon de novo transcription and translation of the polypeptide. Importantly, we report that the GDNF-mediated positive autoregulatory feedback loop accounts for the long-lasting inhibitory actions of GDNF in the VTA on excessive alcohol consumption. Specifically, the long-lasting suppressive effects of a single rGDNF infusion into the VTA on excessive alcohol consumption were prevented when protein synthesis was inhibited, as well as when the upregulation of GDNF expression was prevented using short hairpin RNA to focally knock down GDNF mRNA in the VTA. Our results could have implications for the development of long-lasting treatments for disorders in which GDNF has a beneficial role, including drug addiction, chronic stress and Parkinson's disease.
Keywords: GDNF; addiction; alcohol; growth factors; ventral tegmental area.
Figures

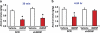
References
-
- Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–1132. - PubMed
-
- Airaksinen MS, Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci. 2002;3:383–394. - PubMed
-
- Barroso-Chinea P, Cruz-Muros I, Aymerich MS, Rodriguez-Diaz M, Afonso-Oramas D, Lanciego JL, et al. Striatal expression of GDNF and differential vulnerability of midbrain dopaminergic cells. Eur J Neurosci. 2005;21:1815–1827. - PubMed
-
- Pochon NA, Menoud A, Tseng JL, Zurn AD, Aebischer P. Neuronal GDNF expression in the adult rat nervous system identified by in situ hybridization. Eur J Neurosci. 1997;9:463–471. - PubMed

