A molecular mechanism for modulating plasma Zn speciation by fatty acids
- PMID: 22239162
- PMCID: PMC3285120
- DOI: 10.1021/ja210496n
A molecular mechanism for modulating plasma Zn speciation by fatty acids
Abstract
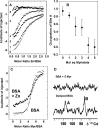
Albumin transports both fatty acids and zinc in plasma. Competitive binding studied by isothermal titration calorimetry revealed that physiologically relevant levels of fatty acids modulate the Zn-binding capacity of albumin, with far-reaching implications for biological zinc speciation. The molecular mechanism for this effect is likely due to a large conformational change elicited by fatty acid binding to a high-affinity interdomain site that disrupts at least one Zn site. Albumin may be a molecular device to "translate" certain aspects of the organismal energy state into global zinc signals.
© 2012 American Chemical Society
Figures
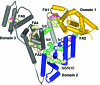



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

