Development and application of a high-throughput microneutralization assay: lack of xenotropic murine leukemia virus-related virus and/or murine leukemia virus detection in blood donors
- PMID: 22239212
- PMCID: PMC3299481
- DOI: 10.1111/j.1537-2995.2011.03519.x
Development and application of a high-throughput microneutralization assay: lack of xenotropic murine leukemia virus-related virus and/or murine leukemia virus detection in blood donors
Abstract
Background: Xenotropic murine leukemia virus (MLV)-related virus (XMRV) and other related MLVs have been described with chronic fatigue syndrome and certain types of prostate cancer. In addition, prevalence rates as high as 7% have been reported in blood donors, raising the risk of transfusion-related transmission. Several laboratories have utilized microneutralization assays as a surrogate marker for detection of anti-MLV serologic responses--with up to 25% of prostate cancer patients reported to harbor neutralizing antibody responses.
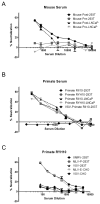
Study design and methods: We developed a high-throughput microneutralization assay for research studies on blood donors using retroviral vectors pseudotyped with XMRV-specific envelopes. Infection with these pseudotypes was neutralized by sera from both macaques and mice challenged with XMRV, but not preimmune serum. A total of 354 plasma samples from blood donors in the Reno/Tahoe area were screened for neutralization.
Results: A total of 6.5% of donor samples gave moderate neutralization of XMRV, but not control pseudotypes. However, further testing by Western blot revealed no evidence of antibodies against MLVs in any of these samples. Furthermore, no evidence of infectious virus or viral nucleic acid was observed.
Conclusion: A microneutralization assay was developed for detection of XMRV and can be applied in a high-throughput format for large-scale studies. Although a proportion of blood donors demonstrated the ability to block XMRV envelope-mediated infection, we found no evidence that this inhibition was mediated by specific antibodies elicited by exposure to XMRV or MLV. It is likely that this moderate neutralization is mediated through another, nonspecific mechanism.
© 2012 American Association of Blood Banks.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




Similar articles
-
Seroprevalence of xenotropic murine leukemia virus-related virus in normal and retrovirus-infected blood donors.Transfusion. 2012 Feb;52(2):307-16. doi: 10.1111/j.1537-2995.2011.03395.x. Epub 2011 Oct 24. Transfusion. 2012. PMID: 22023235
-
No association of xenotropic murine leukemia virus-related virus with prostate cancer or chronic fatigue syndrome in Japan.Retrovirology. 2011 Mar 17;8:20. doi: 10.1186/1742-4690-8-20. Retrovirology. 2011. PMID: 21414229 Free PMC article.
-
Xenotropic murine leukemia virus-related virus does not pose a risk to blood recipient safety.Transfusion. 2012 Feb;52(2):298-306. doi: 10.1111/j.1537-2995.2011.03450.x. Epub 2011 Nov 21. Transfusion. 2012. PMID: 22098340
-
Xenotropic Murine Leukemia Virus-Related Virus (XMRV) and the Safety of the Blood Supply.Clin Microbiol Rev. 2016 Oct;29(4):749-57. doi: 10.1128/CMR.00086-15. Clin Microbiol Rev. 2016. PMID: 27358491 Free PMC article. Review.
-
Distribution of xenotropic murine leukemia virus-related virus (XMRV) infection in chronic fatigue syndrome and prostate cancer.AIDS Rev. 2010 Jul-Sep;12(3):149-52. AIDS Rev. 2010. PMID: 20842203 Review.
Cited by
-
No biological evidence of XMRV in blood or prostatic fluid from prostate cancer patients.PLoS One. 2012;7(5):e36073. doi: 10.1371/journal.pone.0036073. Epub 2012 May 16. PLoS One. 2012. PMID: 22615749 Free PMC article.
-
Ebola Virus Neutralizing Antibodies Detectable in Survivors of theYambuku, Zaire Outbreak 40 Years after Infection.J Infect Dis. 2018 Jan 4;217(2):223-231. doi: 10.1093/infdis/jix584. J Infect Dis. 2018. PMID: 29253164 Free PMC article.
-
Serologic Prevalence of Ebola Virus in Equatorial Africa.Emerg Infect Dis. 2019 May;25(5):911-918. doi: 10.3201/eid2505.180115. Emerg Infect Dis. 2019. PMID: 31002071 Free PMC article.
-
Emerging infectious agents and the nation's blood supply: responding to potential threats in the 21st century.Transfusion. 2013 Feb;53(2):438-54. doi: 10.1111/j.1537-2995.2012.03742.x. Epub 2012 Jun 13. Transfusion. 2013. PMID: 22690676 Free PMC article. No abstract available.
-
No evidence for xenotropic murine leukemia-related virus infection in Sweden using internally controlled multiepitope suspension array serology.Clin Vaccine Immunol. 2012 Sep;19(9):1399-410. doi: 10.1128/CVI.00391-12. Epub 2012 Jul 11. Clin Vaccine Immunol. 2012. PMID: 22787191 Free PMC article.
References
-
- Fischer N, Hellwinkel O, Schulz C, Chun FK, Huland H, Aepfelbacher M, Schlomm T. Prevalence of human gammaretrovirus XMRV in sporadic prostate cancer. J Clin Virol. 2008;43:277–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

