A complex of methylthioadenosine/S-adenosylhomocysteine nucleosidase, transition state analogue, and nucleophilic water identified by mass spectrometry
- PMID: 22239413
- PMCID: PMC3268516
- DOI: 10.1021/ja211176q
A complex of methylthioadenosine/S-adenosylhomocysteine nucleosidase, transition state analogue, and nucleophilic water identified by mass spectrometry
Abstract
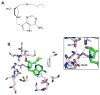
An enzyme-stabilized nucleophilic water molecule has been implicated at the transition state of Escherichia coli methylthioadenosine nucleosidase (EcMTAN) by transition state analysis and crystallography. We analyzed the EcMTAN mass in complex with a femtomolar transition state analogue to determine whether the inhibitor and nucleophilic water could be detected in the gas phase. EcMTAN-inhibitor and EcMTAN-inhibitor-nucleophilic water complexes were identified by high-resolution mass spectrometry under nondenaturing conditions. The enzyme-inhibitor-water complex is sufficiently stable to exist in the gas phase.
© 2012 American Chemical Society
Figures


Similar articles
-
Structural rationale for the affinity of pico- and femtomolar transition state analogues of Escherichia coli 5'-methylthioadenosine/S-adenosylhomocysteine nucleosidase.J Biol Chem. 2005 May 6;280(18):18274-82. doi: 10.1074/jbc.M414471200. Epub 2005 Mar 3. J Biol Chem. 2005. PMID: 15746096
-
Structure of Staphylococcus aureus 5'-methylthioadenosine/S-adenosylhomocysteine nucleosidase.Acta Crystallogr Sect F Struct Biol Cryst Commun. 2008 May 1;64(Pt 5):343-50. doi: 10.1107/S1744309108009275. Epub 2008 Apr 30. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2008. PMID: 18453700 Free PMC article.
-
Structure of Escherichia coli 5'-methylthioadenosine/ S-adenosylhomocysteine nucleosidase inhibitor complexes provide insight into the conformational changes required for substrate binding and catalysis.J Biol Chem. 2003 Mar 7;278(10):8761-70. doi: 10.1074/jbc.M210836200. Epub 2002 Dec 20. J Biol Chem. 2003. PMID: 12496243
-
Metabolic characteristics and importance of the universal methionine salvage pathway recycling methionine from 5'-methylthioadenosine.IUBMB Life. 2009 Dec;61(12):1132-42. doi: 10.1002/iub.278. IUBMB Life. 2009. PMID: 19946895 Review.
-
Trends in the biochemical pharmacology of 5'-deoxy-5'-methylthioadenosine.Biochem Pharmacol. 1982 Feb 1;31(3):277-88. doi: 10.1016/0006-2952(82)90171-x. Biochem Pharmacol. 1982. PMID: 6803807 Review. No abstract available.
Cited by
-
Enzymatic Transition States and Drug Design.Chem Rev. 2018 Nov 28;118(22):11194-11258. doi: 10.1021/acs.chemrev.8b00369. Epub 2018 Oct 18. Chem Rev. 2018. PMID: 30335982 Free PMC article. Review.
-
Salmonella enterica MTAN at 1.36 Å resolution: a structure-based design of tailored transition state analogs.Structure. 2013 Jun 4;21(6):963-74. doi: 10.1016/j.str.2013.04.009. Epub 2013 May 16. Structure. 2013. PMID: 23685211 Free PMC article.
-
Immucillins in Infectious Diseases.ACS Infect Dis. 2018 Feb 9;4(2):107-117. doi: 10.1021/acsinfecdis.7b00172. Epub 2017 Dec 5. ACS Infect Dis. 2018. PMID: 29151351 Free PMC article. Review.
-
Transition States and transition state analogue interactions with enzymes.Acc Chem Res. 2015 Apr 21;48(4):1032-9. doi: 10.1021/acs.accounts.5b00002. Epub 2015 Apr 7. Acc Chem Res. 2015. PMID: 25848811 Free PMC article.
-
Catalytic site cooperativity in dimeric methylthioadenosine nucleosidase.Biochemistry. 2014 Mar 11;53(9):1527-35. doi: 10.1021/bi401589n. Epub 2014 Feb 21. Biochemistry. 2014. PMID: 24502544 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

