Chemoenzymatic synthesis of uridine diphosphate-GlcNAc and uridine diphosphate-GalNAc analogs for the preparation of unnatural glycosaminoglycans
- PMID: 22239739
- PMCID: PMC3272127
- DOI: 10.1021/jo202322k
Chemoenzymatic synthesis of uridine diphosphate-GlcNAc and uridine diphosphate-GalNAc analogs for the preparation of unnatural glycosaminoglycans
Abstract
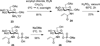
Eight N-acetylglucosamine-1-phosphate and N-acetylgalactosamine-1-phosphate analogs have been synthesized chemically and were tested for their recognition by the GlmU uridyltransferase enzyme. Among these, only substrates that have an amide linkage to the C-2 nitrogen were transferred by GlmU to afford their corresponding uridine diphosphate(UDP)-sugar nucleotides. Resin-immobilized GlmU showed comparable activity to nonimmobilized GlmU and provides a more facile final step in the synthesis of an unnatural UDP-donor. The synthesized unnatural UDP-donors were tested for their activity as substrates for glycosyltransferases in the preparation of unnatural glycosaminoglycans in vitro. A subset of these analogs was useful as donors, increasing the synthetic repertoire for these medically important polysaccharides.
Figures







References
-
- van Heijenoort J. Glycobiology. 2001;11(3):25R–36R. - PubMed
-
- Wu AM. Neurochem. Res. 2002;27(7–8):593–600. - PubMed
-
- Capila I, Linhardt RJ. Angew. Chem. Int. Edit. 2002;41(3):391–412. - PubMed
-
- Watkins WM. Carbohyd. Res. 1986;149(1):1–12. - PubMed
-
- Sismey-Ragatz AE, Green DE, Otto NJ, Rejzek M, Field RA, DeAngelis PL. J. Biol. Chem. 2007;282(39):28321–28327. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

