The paradox of muscle hypertrophy in muscular dystrophy
- PMID: 22239881
- PMCID: PMC5951392
- DOI: 10.1016/j.pmr.2011.11.014
The paradox of muscle hypertrophy in muscular dystrophy
Abstract
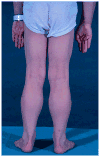
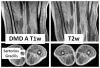
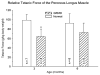
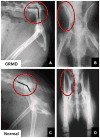
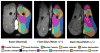
Mutations in the dystrophin gene cause Duchenne and Becker muscular dystrophy in humans and syndromes in mice, dogs, and cats. Affected humans and dogs have progressive disease that leads primarily to muscle atrophy. Mdx mice progress through an initial phase of muscle hypertrophy followed by atrophy. Cats have persistent muscle hypertrophy. Hypertrophy in humans has been attributed to deposition of fat and connective tissue (pseudohypertrophy). Increased muscle mass (true hypertrophy) has been documented in animal models. Muscle hypertrophy can exaggerate postural instability and joint contractures. Deleterious consequences of muscle hypertrophy should be considered when developing treatments for muscular dystrophy.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
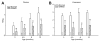

References
-
- Tyler KL. Origins and early descriptions of “Duchenne muscular dystrophy”. Muscle Nerve. 2003;28:402–22. - PubMed
-
- Hoffman EP, Brown RH, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1998;51:919–28. - PubMed
-
- Malhotra S, Hart K, Klamut H, et al. Frame-shift deletions in patients with Duchenne and Becker muscular dystrophy. Science. 1988;242:755–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

