Molecular imaging of bone marrow mononuclear cell survival and homing in murine peripheral artery disease
- PMID: 22239892
- PMCID: PMC3638034
- DOI: 10.1016/j.jcmg.2011.07.011
Molecular imaging of bone marrow mononuclear cell survival and homing in murine peripheral artery disease
Abstract
Objectives: This study aims to provide insight into cellular kinetics using molecular imaging after different transplantation methods of bone marrow-derived mononuclear cells (MNCs) in a mouse model of peripheral artery disease (PAD).
Background: MNC therapy is a promising treatment for PAD. Although clinical translation has already been established, there is a lack of knowledge about cell behavior after transplantation and about the mechanism whereby MNC therapy might ameliorate complaints of PAD.
Methods: MNCs were isolated from F6 transgenic mice (FVB background) that express firefly luciferase (Fluc) and green fluorescence protein (GFP). Male FVB and C57Bl6 mice (n = 50) underwent femoral artery ligation and were randomized into 4 groups receiving the following: 1) single intramuscular (IM) injection of 2 × 10(6) MNCs; 2) 4 weekly IM injections of 5 × 10(5) MNCs; 3) 2 × 10(6) MNCs intravenously; and 4) phosphate-buffered saline as control. Cells were characterized by flow cytometry and in vitro bioluminescence imaging (BLI). Cell survival, proliferation, and migration were monitored by in vivo BLI, which was validated by ex vivo BLI, post-mortem immunohistochemistry, and flow cytometry. Paw perfusion and neovascularization was measured with laser Doppler perfusion imaging (LDPI) and histology, respectively.
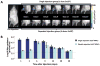
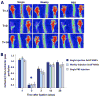
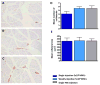
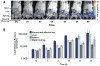
Results: In vivo BLI revealed near-complete donor cell death 4 weeks after IM transplantation. After intravenous transplantation, BLI revealed that cells migrated to the injured area in the limb, as well as to the liver, spleen, and bone marrow. Ex vivo BLI showed presence of MNCs in the scar tissue and adductor muscle. However, no significant effects on neovascularization were observed, as monitored by LDPI and histology.
Conclusions: This is one of the first studies to assess kinetics of transplanted MNCs in PAD using in vivo molecular imaging. MNC survival is short-lived, MNCs do not preferentially home to injured areas, and MNCs do not significantly stimulate perfusion in this particular model.
Copyright © 2012 American College of Cardiology Foundation. Published by Elsevier Inc. All rights reserved.
Figures


Comment in
-
Tracking cell therapy: bioluminescence lighting the way.JACC Cardiovasc Imaging. 2012 Jan;5(1):56-8. doi: 10.1016/j.jcmg.2011.09.017. JACC Cardiovasc Imaging. 2012. PMID: 22239893 No abstract available.
References
-
- Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–146. - PubMed
-
- Belch JJ, Topol EJ, Agnelli G, et al. Critical issues in peripheral arterial disease detection and management: a call to action. Arch Intern Med. 2003;163:884–92. - PubMed
-
- Norgren L, Hiatt WR, Dormandy JA, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) Eur J Vasc Endovasc Surg. 2007;33 (Suppl 1):S1–75. - PubMed
-
- van Weel V, van Tongeren RB, van Hinsbergh VW, van Bockel JH, Quax PH. Vascular growth in ischemic limbs: a review of mechanisms and possible therapeutic stimulation. Ann Vasc Surg. 2008;22:582–97. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

