DNA-capped mesoporous silica nanoparticles as an ion-responsive release system to determine the presence of mercury in aqueous solutions
- PMID: 22240146
- PMCID: PMC3806059
- DOI: 10.1021/ac202993p
DNA-capped mesoporous silica nanoparticles as an ion-responsive release system to determine the presence of mercury in aqueous solutions
Abstract
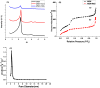
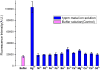
We have developed DNA-functionalized silica nanoparticles for the rapid, sensitive, and selective detection of mercuric ion (Hg(2+)) in aqueous solution. Two DNA strands were designed to cap the pore of dye-trapped silica nanoparticles. In the presence of ppb level Hg(2+), the two DNA strands are dehybridized to uncap the pore, releasing the dye cargo with detectable enhancements of fluorescence signal. This method enables rapid (less than 20 min) and sensitive (limit of detection, LOD, 4 ppb) detection, and it was also able to discriminate Hg(2+) from twelve other environmentally relevant metal ions. The superior properties of the as-designed DNA-functionalized silica nanoparticles can be attributed to the large loading capacity and highly ordered pore structure of mesoporous silica nanoparticles, as well as the selective binding of thymine-rich DNA with Hg(2+) . Our design serves as a new prototype for metal-ion sensing systems, and it also has promising potential for detection of various targets in stimulus-release systems.
Figures

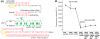
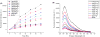


References
-
- Li Tao, S D, Wang Erkang. Label-Free Colorimetric Detection of Aqueous Mercury Ion (Hg2+) Using Hg2+-Modulated G-Quadruplex-Based DNAzymes. Analytical Chemistry. 2009;81:2144–2149. - PubMed
-
- Dave Neeshma, M YC, Jimmy Huang PoJung, Brendan D, Smith, Liu Juewen. Regenerable DNA-Functionalized Hydrogels for Ultrasensitive, Instrument-Free Mercury(II) Detection and Removal in Water. J Am Chem Soc. 2010;132:12668–12673. - PubMed
-
- Nolan E, Lippard S. Tools and Tactics for the Optical Detection of Mercuric Ion. Chemical Review. 2008;108:3433–3480. - PubMed
-
- Chiang C, Huang C, Liu C, Chang H. Oligonucleotide-Based Fluorescence Probe for Sensitive and Selective Detection of Mercury(II) in Aqueous Solution. Analytical Chemistry. 2008;80:3716–3721. - PubMed
-
- Lee J, Mirkin CA. Chip-Based Scanometric Detection of Mercuric Ion Using DNA-Functionalized Gold Nanoparticles. Analytical Chemistry. 2008;80:6805–6808. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

