Combination of a fusogenic glycoprotein, pro-drug activation and oncolytic HSV as an intravesical therapy for superficial bladder cancer
- PMID: 22240799
- PMCID: PMC3273343
- DOI: 10.1038/bjc.2011.577
Combination of a fusogenic glycoprotein, pro-drug activation and oncolytic HSV as an intravesical therapy for superficial bladder cancer
Abstract
Background: There are still no effective treatments for superficial bladder cancer (SBC)/non-muscle invasive bladder cancer. Following treatment, 20% of patients still develop metastatic disease. Superficial bladder cancer is often multifocal, has high recurrences after surgical resection and recurs after intravesical live Bacillus Calmette-Guérin. Oncovex(GALV/CD), an oncolytic herpes simplex virus-1, has shown enhanced local tumour control by combining oncolysis with the expression of a highly potent pro-drug activating gene and the fusogenic glycoprotein.
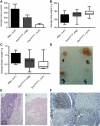
Methods: In vitro fusion/prodrug/apoptotic cell-based assays. In vivo orthotopic bladder tumour model, visualised by computed microtomography.
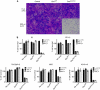
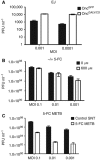
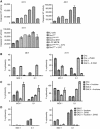
Results: Treatment of seven human bladder carcinoma cell lines with the virus resulted in tumour cell killing through oncolysis, pro-drug activation and glycoprotein fusion. Oncovex(GALV/CD) and mitomycin C showed a synergistic effect, whereas the co-administration with cisplatin or gemcitabine showed an antagonistic effect in vitro. Transitional cell cancer (TCC) cells follow an apoptotic cell death pathway after infection with Oncovex(GALV/CD) with or without 5-FC. In vivo results showed that intravesical treatment with Oncovex(GALV/CD) + prodrug (5-FC) reduced the average tumour volume by over 95% compared with controls.
Discussion: Our in vitro and in vivo results indicate that Oncovex(GALV/CD) can improve local tumour control within the bladder, and potentially alter its natural history.
Conflict of interest statement
We would also like to declare that Toby Price, and Robert S Coffin work for a commercial company Biovex Inc.
Figures


References
-
- Adam L, Black PC, Kassouf W, Eve B, McConkey D, Munsell MF, Benedict WF, Dinney CP (2007) Adenoviral mediated interferon-alpha 2b gene therapy suppresses the pro-angiogenic effect of vascular endothelial growth factor in superficial bladder cancer. J Urol 177: 1900–1906 - PubMed
-
- Anundi I, de GH (1989) Hypoxic liver cell death: critical Po2 and dependence of viability on glycolysis. Am J Physiol 257: G58–G64 - PubMed
-
- Bateman A, Bullough F, Murphy S, Emiliusen L, Lavillette D, Cosset FL, Cattaneo R, Russell SJ, Vile RG (2000) Fusogenic membrane glycoproteins as a novel class of genes for the local and immune-mediated control of tumor growth. Cancer Res 60: 1492–1497 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

