Deletion of CaMKK2 from the liver lowers blood glucose and improves whole-body glucose tolerance in the mouse
- PMID: 22240810
- PMCID: PMC3275166
- DOI: 10.1210/me.2011-1299
Deletion of CaMKK2 from the liver lowers blood glucose and improves whole-body glucose tolerance in the mouse
Abstract
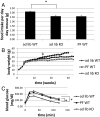
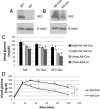
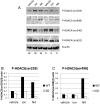
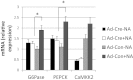
Ca(2+)/calmodulin-dependent protein kinase kinase 2 (CaMKK2) is a member of the Ca(2+)/CaM-dependent protein kinase family that is expressed abundantly in brain. Previous work has revealed that CaMKK2 knockout (CaMKK2 KO) mice eat less due to a central nervous system -signaling defect and are protected from diet-induced obesity, glucose intolerance, and insulin resistance. However, here we show that pair feeding of wild-type mice to match food consumption of CAMKK2 mice slows weight gain but fails to protect from diet-induced glucose intolerance, suggesting that other alterations in CaMKK2 KO mice are responsible for their improved glucose metabolism. CaMKK2 is shown to be expressed in liver and acute, specific reduction of the kinase in the liver of high-fat diet-fed CaMKK2(floxed) mice results in lowered blood glucose and improved glucose tolerance. Primary hepatocytes isolated from CaMKK2 KO mice produce less glucose and have decreased mRNA encoding peroxisome proliferator-activated receptor γ coactivator 1-α and the gluconeogenic enzymes glucose-6-phosphatase and phosphoenolpyruvate carboxykinase, and these mRNA fail to respond specifically to the stimulatory effect of catecholamine in a cell-autonomous manner. The mechanism responsible for suppressed gene induction in CaMKK2 KO hepatocytes may involve diminished phosphorylation of histone deacetylase 5, an event necessary in some contexts for derepression of the peroxisome proliferator-activated receptor γ coactivator 1-α promoter. Hepatocytes from CaMKK2 KO mice also show increased rates of de novo lipogenesis and fat oxidation. The changes in fat metabolism observed correlate with steatotic liver and altered acyl carnitine metabolomic profiles in CaMKK2 KO mice. Collectively, these results are consistent with suppressed catecholamine-induced induction of gluconeogenic gene expression in CaMKK2 KO mice that leads to improved whole-body glucose homeostasis despite the presence of increased hepatic fat content.
Figures


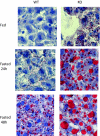

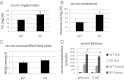
References
-
- Muoio DM, Newgard CB. 2008. Mechanisms of disease: molecular and metabolic mechanisms of insulin resistance and β-cell failure in type 2 diabetes. Nat Rev Mol Cell Biol 9:193–205 - PubMed
-
- Beck-Nielsen H, Hother-Nielsen O, Staehr P. 2002. Is hepatic glucose production increased in type 2 diabetes mellitus? Curr Diab Rep 2:231–236 - PubMed
-
- Gaede P, Vedel P, Parving HH, Pedersen O. 1999. Intensified multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: the Steno type 2 randomised study. Lancet 353:617–622 - PubMed
-
- Jeng CY, Sheu WH, Fuh MM, Chen YD, Reaven GM. 1994. Relationship between hepatic glucose production and fasting plasma glucose concentration in patients with NIDDM. Diabetes 43:1440–1444 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

