Genetics researchers' and IRB professionals' attitudes toward genetic research review: a comparative analysis
- PMID: 22241102
- PMCID: PMC3448270
- DOI: 10.1038/gim.2011.57
Genetics researchers' and IRB professionals' attitudes toward genetic research review: a comparative analysis
Abstract
Purpose: Genetic research involving human participants can pose challenging questions related to ethical and regulatory standards for research oversight. However, few empirical studies describe how genetic researchers and institutional review board (IRB) professionals conceptualize ethical issues in genetic research or where common ground might exist.
Methods: Parallel online surveys collected information from human genetic researchers (n = 351) and IRB professionals (n = 208) regarding their views about human participant oversight for genetic protocols.
Results: A range of opinions were observed within groups on most issues. In both groups, a minority thought it likely that people would be harmed by participation in genetic research or identified from coded genetic data. A majority of both groups agreed that reconsent should be required for four of the six scenarios presented. Statistically significant differences were observed between groups on some issues, with more genetic researcher respondents trusting the confidentiality of coded data, fewer expecting harms from reidentification, and fewer considering reconsent necessary in certain scenarios.
Conclusion: The range of views observed within and between IRB and genetic researcher groups highlights the complexity and unsettled nature of many ethical issues in genome research. Our findings also identify areas where researcher and IRB views diverge and areas of common ground.
Figures
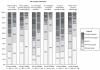
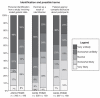
References
-
- Code of Federal Regulations CFR. 2005;45:46.
-
- Jonsen AR. The Birth of Bioethics. Oxford University Press; New York: 1998.
-
- National Institutes of Health [Accessed 6 October 2010];Human Subjects’ Protections. http://www. bioethics.nih.gov/research/protection.shtml.
-
- Greely HT. The uneasy ethical and legal underpinnings of large-scale genomic biobanks. Annu Rev Genomics Hum Genet. 2007;8:343–364. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

