Argininosuccinate lyase deficiency
- PMID: 22241104
- PMCID: PMC3709024
- DOI: 10.1038/gim.2011.1
Argininosuccinate lyase deficiency
Abstract
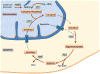
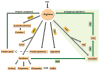
The urea cycle consists of six consecutive enzymatic reactions that convert waste nitrogen into urea. Deficiencies of any of these enzymes of the cycle result in urea cycle disorders (UCDs), a group of inborn errors of hepatic metabolism that often result in life-threatening hyperammonemia. Argininosuccinate lyase (ASL) catalyzes the fourth reaction in this cycle, resulting in the breakdown of argininosuccinic acid to arginine and fumarate. ASL deficiency (ASLD) is the second most common UCD, with a prevalence of ~1 in 70,000 live births. ASLD can manifest as either a severe neonatal-onset form with hyperammonemia within the first few days after birth or as a late-onset form with episodic hyperammonemia and/or long-term complications that include liver dysfunction, neurocognitive deficits, and hypertension. These long-term complications can occur in the absence of hyperammonemic episodes, implying that ASL has functions outside of its role in ureagenesis and the tissue-specific lack of ASL may be responsible for these manifestations. The biochemical diagnosis of ASLD is typically established with elevation of plasma citrulline together with elevated argininosuccinic acid in the plasma or urine. Molecular genetic testing of ASL and assay of ASL enzyme activity are helpful when the biochemical findings are equivocal. However, there is no correlation between the genotype or enzyme activity and clinical outcome. Treatment of acute metabolic decompensations with hyperammonemia involves discontinuing oral protein intake, supplementing oral intake with intravenous lipids and/or glucose, and use of intravenous arginine and nitrogen-scavenging therapy. Dietary restriction of protein and dietary supplementation with arginine are the mainstays in long-term management. Orthotopic liver transplantation (OLT) is best considered only in patients with recurrent hyperammonemia or metabolic decompensations resistant to conventional medical therapy.
Figures
References
-
- SW, Brusilow ALH. The Metabolic and Molecular Bases of Inherited Disease. New York: McGraw-Hill; 2001.
-
- Brusilow SW, Maestri NE. Urea cycle disorders: diagnosis, pathophysiology, and therapy. Adv Pediatr. 1996;43:127–170. - PubMed
-
- Brusilow SW, Horwich AL. Online Metabolic and Molecular Basis of Inherited Disease. 2009
-
- Allan JD, Cusworth DC, Dent CE, Wilson VK. A disease, probably hereditary characterised by severe mental deficiency and a constant gross abnormality of aminoacid metabolism. Lancet. 1958 Jan 25;1(7013):182–187. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K01 RR000188/RR/NCRR NIH HHS/United States
- R01 GM090310/GM/NIGMS NIH HHS/United States
- T32 GM007526/GM/NIGMS NIH HHS/United States
- R01 DK054450/DK/NIDDK NIH HHS/United States
- DK081735/DK/NIDDK NIH HHS/United States
- RR00188/RR/NCRR NIH HHS/United States
- GM07526/GM/NIGMS NIH HHS/United States
- RR19453/RR/NCRR NIH HHS/United States
- U54 RR019453/RR/NCRR NIH HHS/United States
- K08 DK081735/DK/NIDDK NIH HHS/United States
- DK54450/DK/NIDDK NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- GM90310/GM/NIGMS NIH HHS/United States
- M01 RR000188/RR/NCRR NIH HHS/United States
- U54 HD061221/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources

