Human protein arginine methyltransferase 7 (PRMT7) is a type III enzyme forming ω-NG-monomethylated arginine residues
- PMID: 22241471
- PMCID: PMC3318701
- DOI: 10.1074/jbc.M111.336271
Human protein arginine methyltransferase 7 (PRMT7) is a type III enzyme forming ω-NG-monomethylated arginine residues
Abstract
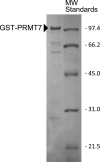
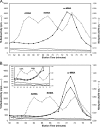
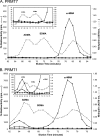
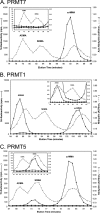
Full-length human protein arginine methyltransferase 7 (PRMT7) expressed as a fusion protein in Escherichia coli was initially found to generate only ω-N(G)-monomethylated arginine residues in small peptides, suggesting that it is a type III enzyme. A later study, however, characterized fusion proteins of PRMT7 expressed in bacterial and mammalian cells as a type II/type I enzyme, capable of producing symmetrically dimethylated arginine (type II activity) as well as small amounts of asymmetric dimethylarginine (type I activity). We have sought to clarify the enzymatic activity of human PRMT7. We analyzed the in vitro methylation products of a glutathione S-transferase (GST)-PRMT7 fusion protein with robust activity using a variety of arginine-containing synthetic peptides and protein substrates, including a GST fusion with the N-terminal domain of fibrillarin (GST-GAR), myelin basic protein, and recombinant human histones H2A, H2B, H3, and H4. Regardless of the methylation reaction conditions (incubation time, reaction volume, and substrate concentration), we found that PRMT7 only produces ω-N(G)-monomethylarginine with these substrates. In control experiments, we showed that mammalian GST-PRMT1 and Myc-PRMT5 were, unlike PRMT7, able to dimethylate both peptide P-SmD3 and SmB/D3 to give the expected asymmetric and symmetric products, respectively. These experiments show that PRMT7 is indeed a type III human methyltransferase capable of forming only ω-N(G)-monomethylarginine, not asymmetric ω-N(G),N(G)-dimethylarginine or symmetric ω-N(G),N(G')-dimethylarginine, under the conditions tested.
Figures

Similar articles
-
PRMT5 (Janus kinase-binding protein 1) catalyzes the formation of symmetric dimethylarginine residues in proteins.J Biol Chem. 2001 Aug 31;276(35):32971-6. doi: 10.1074/jbc.M105412200. Epub 2001 Jun 18. J Biol Chem. 2001. PMID: 11413150
-
Yeast Hsl7 (histone synthetic lethal 7) catalyses the in vitro formation of omega-N(G)-monomethylarginine in calf thymus histone H2A.Biochem J. 2006 May 1;395(3):563-70. doi: 10.1042/BJ20051771. Biochem J. 2006. PMID: 16426232 Free PMC article.
-
Mammalian protein arginine methyltransferase 7 (PRMT7) specifically targets RXR sites in lysine- and arginine-rich regions.J Biol Chem. 2013 Dec 27;288(52):37010-25. doi: 10.1074/jbc.M113.525345. Epub 2013 Nov 18. J Biol Chem. 2013. PMID: 24247247 Free PMC article.
-
PRMT7 as a unique member of the protein arginine methyltransferase family: A review.Arch Biochem Biophys. 2019 Apr 15;665:36-45. doi: 10.1016/j.abb.2019.02.014. Epub 2019 Feb 22. Arch Biochem Biophys. 2019. PMID: 30802433 Free PMC article. Review.
-
Protein arginine methyltransferase 1, a major regulator of biological processes.Biochem Cell Biol. 2024 Apr 1;102(2):106-126. doi: 10.1139/bcb-2023-0212. Epub 2023 Nov 3. Biochem Cell Biol. 2024. PMID: 37922507 Review.
Cited by
-
A unique binding pocket induced by a noncanonical SAH mimic to develop potent and selective PRMT inhibitors.Acta Pharm Sin B. 2023 Dec;13(12):4893-4905. doi: 10.1016/j.apsb.2023.07.022. Epub 2023 Jul 29. Acta Pharm Sin B. 2023. PMID: 38045046 Free PMC article.
-
Protein arginine methyltransferases in renal development, injury, repair, and fibrosis.Front Pharmacol. 2023 Feb 3;14:1123415. doi: 10.3389/fphar.2023.1123415. eCollection 2023. Front Pharmacol. 2023. PMID: 36817133 Free PMC article. Review.
-
Arginine Methylation of the PGC-1α C-Terminus Is Temperature-Dependent.Biochemistry. 2023 Jan 3;62(1):22-34. doi: 10.1021/acs.biochem.2c00363. Epub 2022 Dec 19. Biochemistry. 2023. PMID: 36535003 Free PMC article.
-
PRMT1 Deficiency in Mouse Juvenile Heart Induces Dilated Cardiomyopathy and Reveals Cryptic Alternative Splicing Products.iScience. 2018 Oct 26;8:200-213. doi: 10.1016/j.isci.2018.09.023. Epub 2018 Oct 2. iScience. 2018. PMID: 30321814 Free PMC article.
-
The Biological Axis of Protein Arginine Methylation and Asymmetric Dimethylarginine.Int J Mol Sci. 2019 Jul 6;20(13):3322. doi: 10.3390/ijms20133322. Int J Mol Sci. 2019. PMID: 31284549 Free PMC article. Review.
References
-
- Pahlich S., Zakaryan R. P., Gehring H. (2006) Protein arginine methylation. Cellular functions and methods of analysis. Biochim. Biophys. Acta 1764, 1890–18903 - PubMed
-
- McBride A. E. (2006) in The Enzymes, Third Edition, Protein Methyltransferases (Clarke S. G., Tamanoi F., eds) Vol. 24, pp. 51–103, Academic Press, Burlington, MA
-
- Buhr N., Carapito C., Schaeffer C., Kieffer E., Van Dorsselaer A., Viville S. (2008) Nuclear proteome analysis of undifferentiated mouse embryonic stem and germ cells. Electrophoresis 29, 2381–2390 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

