Undetectable role of oxidative DNA damage in cell cycle, cytotoxic and clastogenic effects of Cr(VI) in human lung cells with restored ascorbate levels
- PMID: 22241526
- PMCID: PMC3382305
- DOI: 10.1093/mutage/ger095
Undetectable role of oxidative DNA damage in cell cycle, cytotoxic and clastogenic effects of Cr(VI) in human lung cells with restored ascorbate levels
Abstract
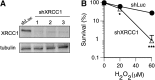
Cultured human cells are invaluable biological models for mechanistic studies of genotoxic chemicals and drugs. Continuing replacement of animals in toxicity testing will further increase the importance of in vitro cell systems, which should accurately reproduce key in vivo characteristics of toxicants such as their profiles of metabolites and DNA lesions. In this work, we examined how a common severe deficiency of cultured cells in ascorbate (Asc) impacts the formation of oxidative DNA damage by hexavalent chromium (chromate). Cr(VI) is reductively activated inside the cells by both Asc and small thiols but with different rates and spectra of intermediates and DNA adducts. We found that Cr(VI) exposure of H460 human lung epithelial cells in standard culture (<0.01 mM cellular Asc) induced biologically significant amounts of oxidative DNA damage. Inhibition of oxidative damage repair in these cells by stable XRCC1 knockdown strongly enhanced cytotoxic effects of Cr(VI) and led to depletion of cells from G(1) and accumulation in S and G(2) phases. However, restoration of physiological levels of Asc (≈ 1 mM) completely eliminated Cr(VI) hypersensitivity of XRCC1 knockdown. The induction of chromosomal breaks assayed by the micronucleus test in Asc-restored H460, primary human lung fibroblasts, and CHO cells was also unaffected by the XRCC1 status. Centromere-negative (clastogenic) micronuclei accounted for 80-90% of all Cr(VI)-induced micronuclei. Consistent with the micronuclei results, Asc-restored cells also showed no increase in the levels of poly(ADP-ribose), which is a biochemical marker of single-stranded breaks. Asc had no effect on cytotoxicity of O(6)-methylguanine, a lesion produced by direct DNA alkylation. Overall, our results indicate that the presence of physiological levels of Asc strongly suppresses pro-oxidant pathways in Cr(VI) metabolism and that the use of standard cell cultures creates a distorted profile of its genotoxic properties.
Figures


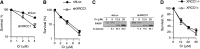

Similar articles
-
Cellular vitamin C increases chromate toxicity via a death program requiring mismatch repair but not p53.Carcinogenesis. 2007 Jul;28(7):1613-20. doi: 10.1093/carcin/bgm031. Epub 2007 Feb 14. Carcinogenesis. 2007. PMID: 17301063
-
Metabolism of Cr(VI) by ascorbate but not glutathione is a low oxidant-generating process.J Trace Elem Med Biol. 2012 Jun;26(2-3):192-6. doi: 10.1016/j.jtemb.2012.04.016. Epub 2012 May 7. J Trace Elem Med Biol. 2012. PMID: 22572042 Free PMC article.
-
Different ATM Signaling in Response to Chromium(VI) Metabolism via Ascorbate and Nonascorbate Reduction: Implications for in Vitro Models and Toxicogenomics.Environ Health Perspect. 2016 Jan;124(1):61-6. doi: 10.1289/ehp.1409434. Epub 2015 May 15. Environ Health Perspect. 2016. PMID: 25977998 Free PMC article.
-
Complexities of chromium carcinogenesis: role of cellular response, repair and recovery mechanisms.Mutat Res. 2003 Dec 10;533(1-2):3-36. doi: 10.1016/j.mrfmmm.2003.09.006. Mutat Res. 2003. PMID: 14643411 Review.
-
Assessment of the mode of action for hexavalent chromium-induced lung cancer following inhalation exposures.Toxicology. 2014 Nov 5;325:160-79. doi: 10.1016/j.tox.2014.08.009. Epub 2014 Aug 28. Toxicology. 2014. PMID: 25174529 Review.
Cited by
-
Toxicological Antagonism among Welding Fume Metals: Inactivation of Soluble Cr(VI) by Iron.Chem Res Toxicol. 2018 Nov 19;31(11):1172-1184. doi: 10.1021/acs.chemrestox.8b00182. Epub 2018 Nov 6. Chem Res Toxicol. 2018. PMID: 30362728 Free PMC article.
-
Carcinogenic Mechanisms of Hexavalent Chromium: From DNA Breaks to Chromosome Instability and Neoplastic Transformation.Curr Environ Health Rep. 2024 Dec;11(4):484-546. doi: 10.1007/s40572-024-00460-9. Epub 2024 Oct 28. Curr Environ Health Rep. 2024. PMID: 39466546 Review.
-
Vitamin C as a Modulator of the Response to Cancer Therapy.Molecules. 2019 Jan 28;24(3):453. doi: 10.3390/molecules24030453. Molecules. 2019. PMID: 30695991 Free PMC article. Review.
-
Role of DNA methylation in cell cycle arrest induced by Cr (VI) in two cell lines.PLoS One. 2013 Aug 6;8(8):e71031. doi: 10.1371/journal.pone.0071031. Print 2013. PLoS One. 2013. PMID: 23940686 Free PMC article.
-
Variation in Extracellular Detoxification Is a Link to Different Carcinogenicity among Chromates in Rodent and Human Lungs.Chem Res Toxicol. 2017 Sep 18;30(9):1720-1729. doi: 10.1021/acs.chemrestox.7b00172. Epub 2017 Aug 20. Chem Res Toxicol. 2017. PMID: 28759204 Free PMC article.
References
-
- Salnikow K, Donald SP, Bruick RK, Zhitkovich A, Phang JM, Kasprzak KS. Depletion of intracellular ascorbate by the carcinogenic metals nickel and cobalt results in the induction of hypoxic stress. J. Biol. Chem. 2004;279:40337–40344. - PubMed
-
- Flashman E, Davies SL, Yeoh KK, Schofield CJ. Investigating the dependence of the hypoxia-inducible factor hydroxylases (factor inhibiting HIF and prolyl hydroxylase domain 2) on ascorbate and other reducing agents. Biochem. J. 2010;427:135–142. - PubMed
-
- Strijbis K, Vaz FM, Distel B. Enzymology of the carnitine biosynthesis pathway. IUBMB Life. 2010;62:357–362. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

