Effect of dual inhibition of apoptosis and autophagy in prostate cancer
- PMID: 22241682
- PMCID: PMC3840901
- DOI: 10.1002/pros.22487
Effect of dual inhibition of apoptosis and autophagy in prostate cancer
Abstract
Purpose: Targeting multiple anti-apoptotic proteins is now possible with the small molecule BH3 domain mimetics such as ABT-737. Given recent studies demonstrating that autophagy is a resistance mechanism to multiple therapeutic agents in the setting of apoptotic inhibition, we hypothesized that hydroxychloroquine (HCQ), an anti-malarial drug that inhibits autophagy, will increase cytotoxicity of ABT-737.
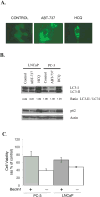
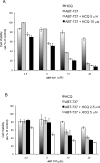
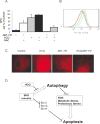
Experimental design: Cytotoxicity of ABT-737 and HCQ was assessed in vitro in PC-3 and LNCaP cells, and in vivo in a xenograft mouse model. The role of autophagy as a resistance mechanism was assessed by siRNA knockdown of the essential autophagy gene beclin1. ROS was measured by flow cytometry, and mitophagy assessed by the mCherry-Parkin reporter.
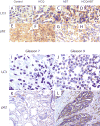
Results: Induction of autophagy by ABT-737 was a mechanism of resistance in prostate cancer cell lines. Therapeutic inhibition of autophagy with HCQ increased cytotoxicity of ABT-737 both in vitro and in vivo. ABT-737 induced LC-3 and decreased p62 expression by immunoblot in cell lines and by immunohistochemistry in tumors in vivo. Assessment of ROS and mitochondria demonstrated that ROS production by ABT-737 and HCQ was a mechanism of cytotoxicity.
Conclusions: We demonstrated that autophagy inhibition with HCQ enhances ABT-737 cytotoxicity in vitro and in vivo, that LC-3 and p62 represent assessable markers in human tissue for future clinical trials, and that ROS induction is a mechanism of cytotoxicity. These results support a new paradigm of dual targeting of apoptosis and autophagy in future clinical studies.
Copyright © 2012 Wiley Periodicals, Inc.
Figures

References
-
- Castilla C, Congregado B, Chinchon D, Torrubia FJ, Japon MA, Saez C. Bcl-xL is overexpressed in hormone-resistant prostate cancer and promotes survival of LNCaP cells via interaction with proapoptotic Bak. Endocrinology. 2006;147:4960–7. - PubMed
-
- Yoshino T, Shiina H, Urakami S, et al. Bcl-2 expression as a predictive marker of hormone-refractory prostate cancer treated with taxane-based chemotherapy. Clin Cancer Res. 2006;12:6116–24. - PubMed
-
- Lin Y, Fukuchi J, Hiipakka RA, Kokontis JM, Xiang J. Up-regulation of Bcl-2 is required for the progression of prostate cancer cells from an androgen-dependent to an androgen-independent growth stage. Cell Res. 2007;17:531–6. - PubMed
-
- Cragg MS, Harris C, Strasser A, Scott CL. Unleashing the power of inhibitors of oncogenic kinases through BH3 mimetics. Nat Rev Cancer. 2009;9:321–6. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

