Ras-related tumorigenesis is suppressed by BNIP3-mediated autophagy through inhibition of cell proliferation
- PMID: 22241963
- PMCID: PMC3257192
- DOI: 10.1593/neo.11888
Ras-related tumorigenesis is suppressed by BNIP3-mediated autophagy through inhibition of cell proliferation
Abstract
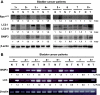
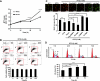
Autophagy plays diverse roles in Ras-related tumorigenesis. H-ras(val12) induces autophagy through multiple signaling pathways including Raf-1/ERK pathway, and various ERK downstream molecules of autophagy have been reported. In this study, Bcl-2/adenovirus E1B 19-kDa-interacting protein 3 (BNIP3) is identified as a downstream transducer of the Ras/Raf/ERK signaling pathway to induce autophagy. BNIP3 was upregulated by H-ras(val12) at the transcriptional level to compete with Beclin 1 for binding with Bcl-2. H-ras(val12)-induced autophagy suppresses cell proliferation demonstrated both in vitro and in vivo by expression of ectopic BNIP3, Atg5, or interference RNA of BNIP3 (siBNIP3) and Atg5 (shAtg5) using mouse NIH3T3 and embryo fibroblast cells. H-ras(val12) induces different autophagic responses depending on the duration of Ras overexpression. After a short time (48 hours) of Ras overexpression, autophagy inhibits cell proliferation. In contrast, a longer time (2 weeks) of Ras overexpression, cell proliferation was enhanced by autophagy. Furthermore, overexpression of mutant Ras, BNIP3, and LC3-II was detected in bladder cancer T24 cells and the tumor parts of 75% of bladder cancer specimens indicating a positive correlation between autophagy and tumorigenesis. Taken together, our mouse model demonstrates a balance between BNIP3-mediated autophagy and H-ras(val12)-induced tumor formation and reveals that H-ras(val12) induces autophagy in a BNIP3-dependent manner, and the threshold of autophagy plays a decisive role in H-ras(val12)-induced tumorigenesis. Our findings combined with others' reports suggest a new therapeutic strategy against Ras-related tumorigenesis by negative or positive regulation of autophagic activity, which is determined by the level of autophagy and tumor progression stages.
Figures




References
-
- Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007;8:741–752. - PubMed
-
- Mizushima N. Autophagy: process and function. Genes Dev. 2007;21:2861–2873. - PubMed
-
- Yoshimori T. Autophagy: a regulated bulk degradation process inside cells. Biochem Biophys Res Commun. 2004;313:453–458. - PubMed
-
- Lamark T, Kirkin V, Dikic I, Johansen T. NBR1 and p62 as cargo receptors for selective autophagy of ubiquitinated targets. Cell Cycle. 2009;8:1986–1990. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
