Inflammasome-dependent pyroptosis and IL-18 protect against Burkholderia pseudomallei lung infection while IL-1β is deleterious
- PMID: 22241982
- PMCID: PMC3248555
- DOI: 10.1371/journal.ppat.1002452
Inflammasome-dependent pyroptosis and IL-18 protect against Burkholderia pseudomallei lung infection while IL-1β is deleterious
Abstract
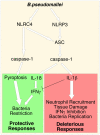
Burkholderia pseudomallei is a Gram-negative bacterium that infects macrophages and other cell types and causes melioidosis. The interaction of B. pseudomallei with the inflammasome and the role of pyroptosis, IL-1β, and IL-18 during melioidosis have not been investigated in detail. Here we show that the Nod-like receptors (NLR) NLRP3 and NLRC4 differentially regulate pyroptosis and production of IL-1β and IL-18 and are critical for inflammasome-mediated resistance to melioidosis. In vitro production of IL-1β by macrophages or dendritic cells infected with B. pseudomallei was dependent on NLRC4 and NLRP3 while pyroptosis required only NLRC4. Mice deficient in the inflammasome components ASC, caspase-1, NLRC4, and NLRP3, were dramatically more susceptible to lung infection with B. pseudomallei than WT mice. The heightened susceptibility of Nlrp3⁻/⁻ mice was due to decreased production of IL-18 and IL-1β. In contrast, Nlrc4⁻/⁻ mice produced IL-1β and IL-18 in higher amount than WT mice and their high susceptibility was due to decreased pyroptosis and consequently higher bacterial burdens. Analyses of IL-18-deficient mice revealed that IL-18 is essential for survival primarily because of its ability to induce IFNγ production. In contrast, studies using IL-1RI-deficient mice or WT mice treated with either IL-1β or IL-1 receptor agonist revealed that IL-1β has deleterious effects during melioidosis. The detrimental role of IL-1β appeared to be due, in part, to excessive recruitment of neutrophils to the lung. Because neutrophils do not express NLRC4 and therefore fail to undergo pyroptosis, they may be permissive to B. pseudomallei intracellular growth. Administration of neutrophil-recruitment inhibitors IL-1ra or the CXCR2 neutrophil chemokine receptor antagonist antileukinate protected Nlrc4⁻/⁻ mice from lethal doses of B. pseudomallei and decreased systemic dissemination of bacteria. Thus, the NLRP3 and NLRC4 inflammasomes have non-redundant protective roles in melioidosis: NLRC4 regulates pyroptosis while NLRP3 regulates production of protective IL-18 and deleterious IL-1β.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

