Inflammatory monocytes and neutrophils are licensed to kill during memory responses in vivo
- PMID: 22241983
- PMCID: PMC3248567
- DOI: 10.1371/journal.ppat.1002457
Inflammatory monocytes and neutrophils are licensed to kill during memory responses in vivo
Abstract
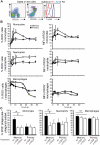
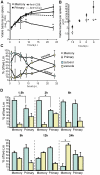
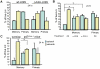
Immunological memory is a hallmark of B and T lymphocytes that have undergone a previous encounter with a given antigen. It is assumed that memory cells mediate better protection of the host upon re-infection because of improved effector functions such as antibody production, cytotoxic activity and cytokine secretion. In contrast to cells of the adaptive immune system, innate immune cells are believed to exhibit a comparable functional effector response each time the same pathogen is encountered. Here, using mice infected by the intracellular bacterium Listeria monocytogenes, we show that during a recall bacterial infection, the chemokine CCL3 secreted by memory CD8+ T cells drives drastic modifications of the functional properties of several populations of phagocytes. We found that inflammatory ly6C+ monocytes and neutrophils largely mediated memory CD8+ T cell bacteriocidal activity by producing increased levels of reactive oxygen species (ROS), augmenting the pH of their phagosomes and inducing antimicrobial autophagy. These events allowed an extremely rapid control of bacterial growth in vivo and accounted for protective immunity. Therefore, our results provide evidence that cytotoxic memory CD8+ T cells can license distinct antimicrobial effector mechanisms of innate cells to efficiently clear pathogens.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
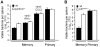



References
-
- Harty JT, Tvinnereim AR, White DW. CD8+ T cell effector mechanisms in resistance to infection. Annu Rev Immunol. 2000;18:275–308. - PubMed
-
- Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. - PubMed
-
- Guidotti LG, Chisari FV. Noncytolytic control of viral infections by the innate and adaptive immune response. Annu Rev Immunol. 2001;19:65–91. - PubMed
-
- Cowley SC, Sedgwick JD, Elkins KL. Differential requirements by CD4+ and CD8+ T cells for soluble and membrane TNF in control of Francisella tularensis live vaccine strain intramacrophage growth. J Immunol. 2007;179:7709–7719. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

