Duox, Flotillin-2, and Src42A are required to activate or delimit the spread of the transcriptional response to epidermal wounds in Drosophila
- PMID: 22242003
- PMCID: PMC3248467
- DOI: 10.1371/journal.pgen.1002424
Duox, Flotillin-2, and Src42A are required to activate or delimit the spread of the transcriptional response to epidermal wounds in Drosophila
Abstract
The epidermis is the largest organ of the body for most animals, and the first line of defense against invading pathogens. A breach in the epidermal cell layer triggers a variety of localized responses that in favorable circumstances result in the repair of the wound. Many cellular and genetic responses must be limited to epidermal cells that are close to wounds, but how this is regulated is still poorly understood. The order and hierarchy of epidermal wound signaling factors are also still obscure. The Drosophila embryonic epidermis provides an excellent system to study genes that regulate wound healing processes. We have developed a variety of fluorescent reporters that provide a visible readout of wound-dependent transcriptional activation near epidermal wound sites. A large screen for mutants that alter the activity of these wound reporters has identified seven new genes required to activate or delimit wound-induced transcriptional responses to a narrow zone of cells surrounding wound sites. Among the genes required to delimit the spread of wound responses are Drosophila Flotillin-2 and Src42A, both of which are transcriptionally activated around wound sites. Flotillin-2 and constitutively active Src42A are also sufficient, when overexpressed at high levels, to inhibit wound-induced transcription in epidermal cells. One gene required to activate epidermal wound reporters encodes Dual oxidase, an enzyme that produces hydrogen peroxide. We also find that four biochemical treatments (a serine protease, a Src kinase inhibitor, methyl-ß-cyclodextrin, and hydrogen peroxide) are sufficient to globally activate epidermal wound response genes in Drosophila embryos. We explore the epistatic relationships among the factors that induce or delimit the spread of epidermal wound signals. Our results define new genetic functions that interact to instruct only a limited number of cells around puncture wounds to mount a transcriptional response, mediating local repair and regeneration.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


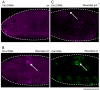
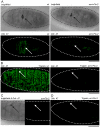
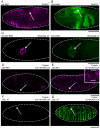
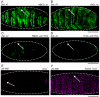
Similar articles
-
Serine proteolytic pathway activation reveals an expanded ensemble of wound response genes in Drosophila.PLoS One. 2013 Apr 24;8(4):e61773. doi: 10.1371/journal.pone.0061773. Print 2013. PLoS One. 2013. PMID: 23637905 Free PMC article.
-
Toll pathway is required for wound-induced expression of barrier repair genes in the Drosophila epidermis.Proc Natl Acad Sci U S A. 2017 Mar 28;114(13):E2682-E2688. doi: 10.1073/pnas.1613917114. Epub 2017 Mar 13. Proc Natl Acad Sci U S A. 2017. PMID: 28289197 Free PMC article.
-
Src kinases and ERK activate distinct responses to Stitcher receptor tyrosine kinase signaling during wound healing in Drosophila.J Cell Sci. 2014 Apr 15;127(Pt 8):1829-39. doi: 10.1242/jcs.143016. Epub 2014 Feb 12. J Cell Sci. 2014. PMID: 24522188
-
Crawling wounded: molecular genetic insights into wound healing from Drosophila larvae.Int J Dev Biol. 2018;62(6-7-8):479-489. doi: 10.1387/ijdb.180085mg. Int J Dev Biol. 2018. PMID: 29938760 Free PMC article. Review.
-
Tension (re)builds: Biophysical mechanisms of embryonic wound repair.Mech Dev. 2017 Apr;144(Pt A):43-52. doi: 10.1016/j.mod.2016.11.004. Epub 2016 Dec 15. Mech Dev. 2017. PMID: 27989746 Review.
Cited by
-
Varroa destructor parasitism has a greater effect on proteome changes than the deformed wing virus and activates TGF-β signaling pathways.Sci Rep. 2019 Jun 28;9(1):9400. doi: 10.1038/s41598-019-45764-1. Sci Rep. 2019. PMID: 31253851 Free PMC article.
-
Dual oxidase: a novel therapeutic target in allergic disease.Br J Pharmacol. 2018 May;175(9):1401-1418. doi: 10.1111/bph.14158. Epub 2018 Mar 15. Br J Pharmacol. 2018. PMID: 29405261 Free PMC article. Review.
-
Wound Healing Insights from Flies and Fish.Cold Spring Harb Perspect Biol. 2022 Nov 1;14(11):a041217. doi: 10.1101/cshperspect.a041217. Cold Spring Harb Perspect Biol. 2022. PMID: 35817511 Free PMC article. Review.
-
Hydrogen peroxide as a damage signal in tissue injury and inflammation: murderer, mediator, or messenger?J Cell Biochem. 2014 Mar;115(3):427-35. doi: 10.1002/jcb.24683. J Cell Biochem. 2014. PMID: 24122865 Free PMC article. Review.
-
Hippo, TGF-β, and Src-MAPK pathways regulate transcription of the upd3 cytokine in Drosophila enterocytes upon bacterial infection.PLoS Genet. 2017 Nov 6;13(11):e1007091. doi: 10.1371/journal.pgen.1007091. eCollection 2017 Nov. PLoS Genet. 2017. PMID: 29108021 Free PMC article.
References
-
- Segre J. Complex redundancy to build a simple epidermal permeability barrier. Curr Opin Cell Biol. 2003;15:776–782. - PubMed
-
- Locke M. The Wigglesworth Lecture: Insects for studying fundamental problems in biology. J Insect Physiol. 2001;47:495–507. - PubMed
-
- Moussian B. Recent advances in understanding mechanisms of insect cuticle differentiation. Insect Biochem Mol Biol. 2010;40:363–375. - PubMed
-
- Martin P, Parkhurst SM. Parallels between tissue repair and embryo morphogenesis. Development. 2004;131:3021–3034. - PubMed
-
- Mace KA, Pearson JC, McGinnis W. An epidermal barrier wound repair pathway in Drosophila is mediated by grainy head. Science. 2005;308:381–385. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

