Interspecific sex in grass smuts and the genetic diversity of their pheromone-receptor system
- PMID: 22242007
- PMCID: PMC3248468
- DOI: 10.1371/journal.pgen.1002436
Interspecific sex in grass smuts and the genetic diversity of their pheromone-receptor system
Erratum in
- PLoS Genet. 2012 Jan;8(1). doi:10.1371/annotation/5febc52b-339c-4f47-82c0-03d417516446
Abstract
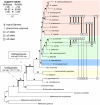
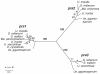
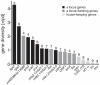
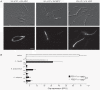
The grass smuts comprise a speciose group of biotrophic plant parasites, so-called Ustilaginaceae, which are specifically adapted to hosts of sweet grasses, the Poaceae family. Mating takes a central role in their life cycle, as it initiates parasitism by a morphological and physiological transition from saprobic yeast cells to pathogenic filaments. As in other fungi, sexual identity is determined by specific genomic regions encoding allelic variants of a pheromone-receptor (PR) system and heterodimerising transcription factors. Both operate in a biphasic mating process that starts with PR-triggered recognition, directed growth of conjugation hyphae, and plasmogamy of compatible mating partners. So far, studies on the PR system of grass smuts revealed diverse interspecific compatibility and mating type determination. However, many questions concerning the specificity and evolutionary origin of the PR system remain unanswered. Combining comparative genetics and biological approaches, we report on the specificity of the PR system and its genetic diversity in 10 species spanning about 100 million years of mating type evolution. We show that three highly syntenic PR alleles are prevalent among members of the Ustilaginaceae, favouring a triallelic determination as the plesiomorphic characteristic of this group. Furthermore, the analysis of PR loci revealed increased genetic diversity of single PR locus genes compared to genes of flanking regions. Performing interspecies sex tests, we detected a high potential for hybridisation that is directly linked to pheromone signalling as known from intraspecies sex. Although the PR system seems to be optimised for intraspecific compatibility, the observed functional plasticity of the PR system increases the potential for interspecific sex, which might allow the hybrid-based genesis of newly combined host specificities.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Convergent evolution of linked mating-type loci in basidiomycete fungi.PLoS Genet. 2019 Sep 6;15(9):e1008365. doi: 10.1371/journal.pgen.1008365. eCollection 2019 Sep. PLoS Genet. 2019. PMID: 31490920 Free PMC article.
-
Fungal Sex: The Basidiomycota.Microbiol Spectr. 2017 Jun;5(3):10.1128/microbiolspec.funk-0046-2016. doi: 10.1128/microbiolspec.FUNK-0046-2016. Microbiol Spectr. 2017. PMID: 28597825 Free PMC article. Review.
-
The Pheromone and Pheromone Receptor Mating-Type Locus Is Involved in Controlling Uniparental Mitochondrial Inheritance in Cryptococcus.Genetics. 2020 Mar;214(3):703-717. doi: 10.1534/genetics.119.302824. Epub 2019 Dec 30. Genetics. 2020. PMID: 31888949 Free PMC article.
-
Genetic Dissection of Sexual Reproduction in a Primary Homothallic Basidiomycete.PLoS Genet. 2016 Jun 21;12(6):e1006110. doi: 10.1371/journal.pgen.1006110. eCollection 2016 Jun. PLoS Genet. 2016. PMID: 27327578 Free PMC article.
-
Sex in smut fungi: Structure, function and evolution of mating-type complexes.Fungal Genet Biol. 2008 Aug;45 Suppl 1:S15-21. doi: 10.1016/j.fgb.2008.04.005. Epub 2008 May 22. Fungal Genet Biol. 2008. PMID: 18501648 Review.
Cited by
-
Evolution of Mating Systems in Basidiomycetes and the Genetic Architecture Underlying Mating-Type Determination in the Yeast Leucosporidium scottii.Genetics. 2015 Sep;201(1):75-89. doi: 10.1534/genetics.115.177717. Epub 2015 Jul 14. Genetics. 2015. PMID: 26178967 Free PMC article.
-
A Tale of Genome Compartmentalization: The Evolution of Virulence Clusters in Smut Fungi.Genome Biol Evol. 2016 Feb 12;8(3):681-704. doi: 10.1093/gbe/evw026. Genome Biol Evol. 2016. PMID: 26872771 Free PMC article.
-
Variation in mate-recognition pheromones of the fungal genus Microbotryum.Heredity (Edinb). 2016 Jan;116(1):44-51. doi: 10.1038/hdy.2015.68. Epub 2015 Aug 26. Heredity (Edinb). 2016. PMID: 26306729 Free PMC article.
-
Patterns of variation at Ustilago maydis virulence clusters 2A and 19A largely reflect the demographic history of its populations.PLoS One. 2014 Jun 2;9(6):e98837. doi: 10.1371/journal.pone.0098837. eCollection 2014. PLoS One. 2014. PMID: 24887029 Free PMC article.
-
Specific phosphoinositide interaction of Jps1 is a key feature during unconventional secretion in Ustilago maydis.J Biol Chem. 2025 Jun;301(6):110215. doi: 10.1016/j.jbc.2025.110215. Epub 2025 May 8. J Biol Chem. 2025. PMID: 40348193 Free PMC article.
References
-
- Weismann A. Zur Frage nach der Vererbung erworbener Eigenschaften. Biologisches Zentralblatt. 1886;6:33–48.
-
- Wilhelm D, Palmer S, Koopman P. Sex determination and gonadal development in mammals. Physiol Rev. 2007;87:1–28. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

