DNA methylation of the gonadal aromatase (cyp19a) promoter is involved in temperature-dependent sex ratio shifts in the European sea bass
- PMID: 22242011
- PMCID: PMC3248465
- DOI: 10.1371/journal.pgen.1002447
DNA methylation of the gonadal aromatase (cyp19a) promoter is involved in temperature-dependent sex ratio shifts in the European sea bass
Abstract
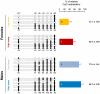
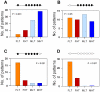
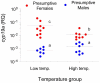
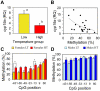
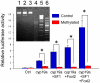
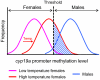
Sex ratio shifts in response to temperature are common in fish and reptiles. However, the mechanism linking temperature during early development and sex ratios has remained elusive. We show in the European sea bass (sb), a fish in which temperature effects on sex ratios are maximal before the gonads form, that juvenile males have double the DNA methylation levels of females in the promoter of gonadal aromatase (cyp19a), the enzyme that converts androgens into estrogens. Exposure to high temperature increased the cyp19a promoter methylation levels of females, indicating that induced-masculinization involves DNA methylation-mediated control of aromatase gene expression, with an observed inverse relationship between methylation levels and expression. Although different CpGs within the sb cyp19a promoter exhibited different sensitivity to temperature, we show that the increased methylation of the sb cyp19a promoter, which occurs in the gonads but not in the brain, is not a generalized effect of temperature. Importantly, these effects were also observed in sexually undifferentiated fish and were not altered by estrogen treatment. Thus, methylation of the sb cyp19a promoter is the cause of the lower expression of cyp19a in temperature-masculinized fish. In vitro, induced methylation of the sb cyp19a promoter suppressed the ability of SF-1 and Foxl2 to stimulate transcription. Finally, a CpG differentially methylated by temperature and adjacent to a Sox transcription factor binding site is conserved across species. Thus, DNA methylation of the aromatase promoter may be an essential component of the long-sought-after mechanism connecting environmental temperature and sex ratios in vertebrate species with temperature-dependent sex determination.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Valenzuela N, Lance V. Temperature-dependent sex determination in vertebrates. Washington: Smithsonian Books; 2004.
-
- Pieau C, Dorizzi M. Oestrogens and temperature-dependent sex determination in reptiles: all is in the gonads. Journal of Endocrinology. 2004;181:367–377. - PubMed
-
- Van Nes S, Andersen O. Temperature effects on sex determination and ontogenetic gene expression of the aromatases cyp19a and cyp19b, and the estrogen receptors esr1 and esr2 in Atlantic halibut (Hippoglossus hippoglossus). Molecular Reproduction and Development. 2006;73:1481–1490. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

