Checkpoints in a yeast differentiation pathway coordinate signaling during hyperosmotic stress
- PMID: 22242015
- PMCID: PMC3252264
- DOI: 10.1371/journal.pgen.1002437
Checkpoints in a yeast differentiation pathway coordinate signaling during hyperosmotic stress
Abstract
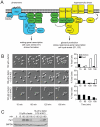
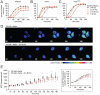
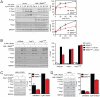
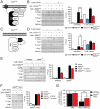
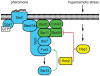
All eukaryotes have the ability to detect and respond to environmental and hormonal signals. In many cases these signals evoke cellular changes that are incompatible and must therefore be orchestrated by the responding cell. In the yeast Saccharomyces cerevisiae, hyperosmotic stress and mating pheromones initiate signaling cascades that each terminate with a MAP kinase, Hog1 and Fus3, respectively. Despite sharing components, these pathways are initiated by distinct inputs and produce distinct cellular behaviors. To understand how these responses are coordinated, we monitored the pheromone response during hyperosmotic conditions. We show that hyperosmotic stress limits pheromone signaling in at least three ways. First, stress delays the expression of pheromone-induced genes. Second, stress promotes the phosphorylation of a protein kinase, Rck2, and thereby inhibits pheromone-induced protein translation. Third, stress promotes the phosphorylation of a shared pathway component, Ste50, and thereby dampens pheromone-induced MAPK activation. Whereas all three mechanisms are dependent on an increase in osmolarity, only the phosphorylation events require Hog1. These findings reveal how an environmental stress signal is able to postpone responsiveness to a competing differentiation signal, by acting on multiple pathway components, in a coordinated manner.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Shared and redundant proteins coordinate signal cross-talk between MAPK pathways in yeast.Mol Biol Cell. 2024 Oct 1;35(10):ar126. doi: 10.1091/mbc.E24-06-0270. Epub 2024 Jul 31. Mol Biol Cell. 2024. PMID: 39083355 Free PMC article.
-
Cdc42-Specific GTPase-Activating Protein Rga1 Squelches Crosstalk between the High-Osmolarity Glycerol (HOG) and Mating Pheromone Response MAPK Pathways.Biomolecules. 2021 Oct 17;11(10):1530. doi: 10.3390/biom11101530. Biomolecules. 2021. PMID: 34680163 Free PMC article.
-
Formation of subnuclear foci is a unique spatial behavior of mating MAPKs during hyperosmotic stress.Cell Rep. 2013 Feb 21;3(2):328-34. doi: 10.1016/j.celrep.2013.01.022. Epub 2013 Feb 14. Cell Rep. 2013. PMID: 23416049 Free PMC article.
-
Heterotrimeric G Protein-coupled Receptor Signaling in Yeast Mating Pheromone Response.J Biol Chem. 2016 Apr 8;291(15):7788-95. doi: 10.1074/jbc.R116.714980. Epub 2016 Feb 23. J Biol Chem. 2016. PMID: 26907689 Free PMC article. Review.
-
Negative feedback-loop mechanisms regulating HOG- and pheromone-MAPK signaling in yeast.Curr Genet. 2020 Oct;66(5):867-880. doi: 10.1007/s00294-020-01089-5. Epub 2020 Jun 20. Curr Genet. 2020. PMID: 32564133 Review.
Cited by
-
Crosstalk and spatiotemporal regulation between stress-induced MAP kinase pathways and pheromone signaling in budding yeast.Cell Cycle. 2020 Jul;19(14):1707-1715. doi: 10.1080/15384101.2020.1779469. Epub 2020 Jun 18. Cell Cycle. 2020. PMID: 32552303 Free PMC article. Review.
-
Network inference reveals novel connections in pathways regulating growth and defense in the yeast salt response.PLoS Comput Biol. 2018 May 8;13(5):e1006088. doi: 10.1371/journal.pcbi.1006088. eCollection 2018 May. PLoS Comput Biol. 2018. PMID: 29738528 Free PMC article.
-
Shared and redundant proteins coordinate signal cross-talk between MAPK pathways in yeast.Mol Biol Cell. 2024 Oct 1;35(10):ar126. doi: 10.1091/mbc.E24-06-0270. Epub 2024 Jul 31. Mol Biol Cell. 2024. PMID: 39083355 Free PMC article.
-
Comparative Analysis of Transmembrane Regulators of the Filamentous Growth Mitogen-Activated Protein Kinase Pathway Uncovers Functional and Regulatory Differences.Eukaryot Cell. 2015 Sep;14(9):868-83. doi: 10.1128/EC.00085-15. Epub 2015 Jun 26. Eukaryot Cell. 2015. PMID: 26116211 Free PMC article.
-
Activator and repressor functions of the Mot3 transcription factor in the osmostress response of Saccharomyces cerevisiae.Eukaryot Cell. 2013 May;12(5):636-47. doi: 10.1128/EC.00037-13. Epub 2013 Feb 22. Eukaryot Cell. 2013. PMID: 23435728 Free PMC article.
References
-
- Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–1912. - PubMed
-
- Qi M, Elion EA. MAP kinase pathways. J Cell Sci. 2005;118:3569–3572. - PubMed
-
- Dohlman HG, Thorner JW. Regulation of G protein-initiated signal transduction in yeast: paradigms and principles. Annu Rev Biochem. 2001;70:703–754. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

