Bidirectional coupling between astrocytes and neurons mediates learning and dynamic coordination in the brain: a multiple modeling approach
- PMID: 22242121
- PMCID: PMC3248449
- DOI: 10.1371/journal.pone.0029445
Bidirectional coupling between astrocytes and neurons mediates learning and dynamic coordination in the brain: a multiple modeling approach
Abstract
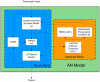
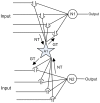
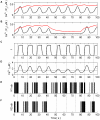
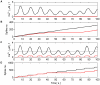
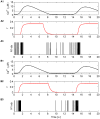
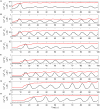
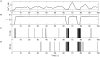
In recent years research suggests that astrocyte networks, in addition to nutrient and waste processing functions, regulate both structural and synaptic plasticity. To understand the biological mechanisms that underpin such plasticity requires the development of cell level models that capture the mutual interaction between astrocytes and neurons. This paper presents a detailed model of bidirectional signaling between astrocytes and neurons (the astrocyte-neuron model or AN model) which yields new insights into the computational role of astrocyte-neuronal coupling. From a set of modeling studies we demonstrate two significant findings. Firstly, that spatial signaling via astrocytes can relay a "learning signal" to remote synaptic sites. Results show that slow inward currents cause synchronized postsynaptic activity in remote neurons and subsequently allow Spike-Timing-Dependent Plasticity based learning to occur at the associated synapses. Secondly, that bidirectional communication between neurons and astrocytes underpins dynamic coordination between neuron clusters. Although our composite AN model is presently applied to simplified neural structures and limited to coordination between localized neurons, the principle (which embodies structural, functional and dynamic complexity), and the modeling strategy may be extended to coordination among remote neuron clusters.
Conflict of interest statement
Figures















References
-
- Kang J, Jiang L, Goldman S, Nedergaard M. Astrocyte-mediated potentiation of inhibitory synaptic transmission. Nat Neurosci. 1998;1:683–692. doi: 10.1038/3684. - DOI - PubMed
-
- Kurosinski P, Gotz J. Glial cells under physiologic and pathologic conditions. Arch Neurol. 2002;59:1524–1528. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

