Stress-induced C/EBP homology protein (CHOP) represses MyoD transcription to delay myoblast differentiation
- PMID: 22242125
- PMCID: PMC3248460
- DOI: 10.1371/journal.pone.0029498
Stress-induced C/EBP homology protein (CHOP) represses MyoD transcription to delay myoblast differentiation
Abstract
When mouse myoblasts or satellite cells differentiate in culture, the expression of myogenic regulatory factor, MyoD, is downregulated in a subset of cells that do not differentiate. The mechanism involved in the repression of MyoD expression remains largely unknown. Here we report that a stress-response pathway repressing MyoD transcription is transiently activated in mouse-derived C2C12 myoblasts growing under differentiation-promoting conditions. We show that phosphorylation of the α subunit of the translation initiation factor 2 (eIF2α) is followed by expression of C/EBP homology protein (CHOP) in some myoblasts. ShRNA-driven knockdown of CHOP expression caused earlier and more robust differentiation, whereas its constitutive expression delayed differentiation relative to wild type myoblasts. Cells expressing CHOP did not express the myogenic regulatory factors MyoD and myogenin. These results indicated that CHOP directly repressed the transcription of the MyoD gene. In support of this view, CHOP associated with upstream regulatory region of the MyoD gene and its activity reduced histone acetylation at the enhancer region of MyoD. CHOP interacted with histone deacetylase 1 (HDAC1) in cells. This protein complex may reduce histone acetylation when bound to MyoD regulatory regions. Overall, our results suggest that the activation of a stress pathway in myoblasts transiently downregulate the myogenic program.
Conflict of interest statement
Figures

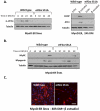
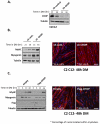
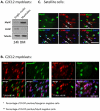
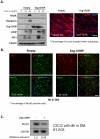


References
-
- Tajbakhsh S. Skeletal muscle stem cells in developmental versus regenerative myogenesis. J Intern Med. 2009;266:372–389. - PubMed
-
- Fuchtbauer EM, Westphal H. MyoD and myogenin are coexpressed in regenerating skeletal muscle of the mouse. Dev Dyn. 1992;193:34–39. - PubMed
-
- Megeney LA, Kablar B, Garrett K, Anderson JE, Rudnicki MA. MyoD is required for myogenic stem cell function in adult skeletal muscle. Genes Dev. 1996;10:1173–1183. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

