Pharmacokinetic predictions for patients with renal impairment: focus on peptides and protein drugs
- PMID: 22242561
- PMCID: PMC3394130
- DOI: 10.1111/j.1365-2125.2012.04172.x
Pharmacokinetic predictions for patients with renal impairment: focus on peptides and protein drugs
Abstract
What is already known about this subject: • Renal impairment may affect the pharmacokinetics of peptide and protein drugs. • Molecular size is a predictor. Small molecules are eliminated by the kidneys, whereas large molecules (>67 kDa) are not. • Urinary recovery of peptide and protein drugs in healthy volunteers is not predictive for pharmacokinetic changes in patients with renal impairment.
What this study adds: • An apparently continuous non-linear relationship between molecular weight and pharmacokinetic alterations as observed in patients with severe renal impairment or end-stage renal disease is described. • Potentially relevant pharmacokinetic changes were found for drugs with a molecular weight below 50 kDa. • Analysis of observed pharmacokinetics in patients with severe renal impairment may be a useful approach, especially when urinary recovery in healthy volunteers is not predictive.
Aim: Drug dosage adjustments in renal impairment are usually based on estimated individual pharmacokinetics. The extent of pharmacokinetic changes in patients with renal impairment must be known for this estimation. If measured data are not available, an estimate based on drug elimination in urine of healthy subjects or patients with normal renal function is commonly made. This is not reliable, however, if renal drug metabolism is involved, as is presumably the case for many peptide and protein drugs. In the present study a new method to predict pharmacokinetic changes for such drugs based on molecular weight was derived.
Methods: Articles reporting measured pharmacokinetics of peptide and protein drugs in patients with severe renal impairment or end-stage renal disease were identified from the scientific literature, the pharmacokinetic parameter values were extracted and a statistical data synthesis was performed. A sigmoid E(max) model was applied and fitted to the data and the prediction error was analyzed.
Results: Overall, 98 peptide and protein drugs were identified. Relevant pharmacokinetic data in patients with renal impairment were found for 21 of these drugs. The average drug clearance was 30% and the average prolongation in half-life was 3.1-fold for low molecular weight peptides or proteins. The median root squared percentage of the prediction error was 18% (drug clearance) and 12% (half-life).
Conclusion: An apparently continuous non-linear relationship between molecular weight and pharmacokinetic alterations in patients with severe renal impairment was found. The derived equations could be used as a rough guide for decisions on drug dosage adjustments in such patients.
© 2012 The Authors. British Journal of Clinical Pharmacology © 2012 The British Pharmacological Society.
Figures
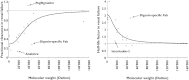
 ) of 3 indicates a three-fold prolongation of half-life in renal failure. The continuous lines represent the fitted sigmoid Emax model (equation 4). The broken lines represent the hypothetical condition of no change
) of 3 indicates a three-fold prolongation of half-life in renal failure. The continuous lines represent the fitted sigmoid Emax model (equation 4). The broken lines represent the hypothetical condition of no changeSimilar articles
-
A GFR-Based Method to Predict the Effect of Renal Impairment on the Exposure or Clearance of Renally Excreted Drugs: A Comparative Study Between a Simple GFR Method and a Physiologically Based Pharmacokinetic Model.Drugs R D. 2020 Dec;20(4):377-387. doi: 10.1007/s40268-020-00327-y. Epub 2020 Nov 4. Drugs R D. 2020. PMID: 33150526 Free PMC article.
-
[Pharmacokinetic changes in renal failure].Presse Med. 2001 Mar 31;30(12):597-604. Presse Med. 2001. PMID: 11317921 Review. French.
-
Pharmacokinetic and pharmacodynamic properties of eptifibatide in subjects with normal or impaired renal function.Clin Ther. 2004 Mar;26(3):390-8. doi: 10.1016/s0149-2918(04)90034-3. Clin Ther. 2004. PMID: 15110131 Clinical Trial.
-
Evaluation of Model-Based Prediction of Pharmacokinetics in the Renal Impairment Population.J Clin Pharmacol. 2018 Mar;58(3):364-376. doi: 10.1002/jcph.1022. Epub 2017 Oct 27. J Clin Pharmacol. 2018. PMID: 29077203
-
Optimization of Protein and Peptide Drugs Based on the Mechanisms of Kidney Clearance.Protein Pept Lett. 2018;25(6):514-521. doi: 10.2174/0929866525666180530122835. Protein Pept Lett. 2018. PMID: 29848260 Review.
Cited by
-
The impact of hepatic and renal function on panitumumab exposures in patients with metastatic RAS wild-type colorectal cancer.Cancer Chemother Pharmacol. 2021 Oct;88(4):665-672. doi: 10.1007/s00280-021-04319-w. Epub 2021 Jul 2. Cancer Chemother Pharmacol. 2021. PMID: 34213592 Free PMC article.
-
Strategic approaches to optimizing peptide ADME properties.AAPS J. 2015 Jan;17(1):134-43. doi: 10.1208/s12248-014-9687-3. Epub 2014 Nov 4. AAPS J. 2015. PMID: 25366889 Free PMC article. Review.
-
Effect of Intrinsic and Extrinsic Factors on the Pharmacokinetics of Antibody-Drug Conjugates (ADCs).Antibodies (Basel). 2021 Oct 15;10(4):40. doi: 10.3390/antib10040040. Antibodies (Basel). 2021. PMID: 34698086 Free PMC article. Review.
-
Pharmacokinetics, distribution, metabolism, and excretion of body-protective compound 157, a potential drug for treating various wounds, in rats and dogs.Front Pharmacol. 2022 Dec 14;13:1026182. doi: 10.3389/fphar.2022.1026182. eCollection 2022. Front Pharmacol. 2022. PMID: 36588717 Free PMC article.
-
Impact of Intrinsic and Extrinsic Factors on the Pharmacokinetics of Peptides: When Is the Assessment of Certain Factors Warranted?Antibodies (Basel). 2021 Dec 21;11(1):1. doi: 10.3390/antib11010001. Antibodies (Basel). 2021. PMID: 35076485 Free PMC article. Review.
References
-
- Dettli L. Drug dosage in renal disease. Clin Pharmacokinet. 1976;1:126–34. - PubMed
-
- Welling PG, Craig WA, Kunin CM. Prediction of drug dosage in patients with renal failure using data derived from normal subjects. Clin Pharmacol Ther. 1975;18:45–52. - PubMed
-
- Pichette V, Leblond FA. Drug metabolism in chronic renal failure. Curr Drug Metab. 2003;4:91–103. - PubMed
-
- Rubenstein AH, Mako ME, Horwitz DL. Insulin and the kidney. Nephron. 1975;15:306–26. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

