Probing structural features of Alzheimer's amyloid-β pores in bilayers using site-specific amino acid substitutions
- PMID: 22242635
- PMCID: PMC3265145
- DOI: 10.1021/bi2017427
Probing structural features of Alzheimer's amyloid-β pores in bilayers using site-specific amino acid substitutions
Abstract
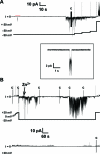
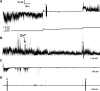
A current hypothesis for the pathology of Alzheimer's disease (AD) proposes that amyloid-β (Aβ) peptides induce uncontrolled, neurotoxic ion flux across cellular membranes. The mechanism of ion flux is not fully understood because no experiment-based Aβ channel structures at atomic resolution are currently available (only a few polymorphic states have been predicted by computational models). Structural models and experimental evidence lend support to the view that the Aβ channel is an assembly of loosely associated mobile β-sheet subunits. Here, using planar lipid bilayers and molecular dynamics (MD) simulations, we show that amino acid substitutions can be used to infer which residues are essential for channel structure. We created two Aβ(1-42) peptides with point mutations: F19P and F20C. The substitution of Phe19 with Pro inhibited channel conductance. MD simulation suggests a collapsed pore of F19P channels at the lower bilayer leaflet. The kinks at the Pro residues in the pore-lining β-strands induce blockage of the solvated pore by the N-termini of the chains. The cysteine mutant is capable of forming channels, and the conductance behavior of F20C channels is similar to that of the wild type. Overall, the mutational analysis of the channel activity performed in this work tests the proposition that the channels consist of a β-sheet rich organization, with the charged/polar central strand containing the mutation sites lining the pore, and the C-terminal strands facing the hydrophobic lipid tails. A detailed understanding of channel formation and its structure should aid studies of drug design aiming to control unregulated Aβ-dependent ion fluxes.
Figures



References
-
- Selkoe D. J. (1991) The molecular pathology of Alzheimer’s disease. Neuron 6, 487–498. - PubMed
-
- Selkoe D. J. (1989) Amyloid β protein precursor and the pathogenesis of Alzheimer’s disease. Cell 58, 611–612. - PubMed
-
- Selkoe D. J. (1989) Aging, amyloid, and Alzheimer’s disease. N. Engl. J. Med. 320, 1484–1487. - PubMed
-
- Ferretti M. T.; Bruno M. A.; Ducatenzeiler A.; Klein W. L.; Cuello A. C. (2011) Intracellular Aβ-oligomers and early inflammation in a model of Alzheimer’s disease. Neurobiol. Aging DOI: 10.1016/j.neurobiolaging.2011.01.007. - PubMed
-
- Klein W. L. (2002) Aβ toxicity in Alzheimer’s disease: Globular oligomers (ADDLs) as new vaccine and drug targets. Neurochem. Int. 41, 345–352. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

