Acentric 2-D ensembles of D-br-A electron-transfer chromophores via vectorial orientation within amphiphilic n-helix bundle peptides for photovoltaic device applications
- PMID: 22242787
- PMCID: PMC3391659
- DOI: 10.1021/la205002f
Acentric 2-D ensembles of D-br-A electron-transfer chromophores via vectorial orientation within amphiphilic n-helix bundle peptides for photovoltaic device applications
Abstract
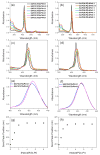
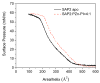
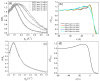
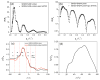
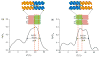
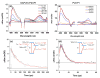
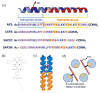
We show that simply designed amphiphilic 4-helix bundle peptides can be utilized to vectorially orient a linearly extended donor-bridge-acceptor (D-br-A) electron transfer (ET) chromophore within its core. The bundle's interior is shown to provide a unique solvation environment for the D-br-A assembly not accessible in conventional solvents and thereby control the magnitudes of both light-induced ET and thermal charge recombination rate constants. The amphiphilicity of the bundle's exterior was employed to vectorially orient the peptide-chromophore complex at a liquid-gas interface, and its ends were tailored for subsequent covalent attachment to an inorganic surface, via a "directed assembly" approach. Structural data, combined with evaluation of the excited state dynamics exhibited by these peptide-chromophore complexes, demonstrate that densely packed, acentrically ordered 2-D monolayer ensembles of such complexes at high in-plane chromophore densities approaching 1/200 Å(2) offer unique potential as active layers in binary heterojunction photovoltaic devices.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

