Assembly and maintenance of nodes of ranvier rely on distinct sources of proteins and targeting mechanisms
- PMID: 22243749
- PMCID: PMC3448493
- DOI: 10.1016/j.neuron.2011.10.016
Assembly and maintenance of nodes of ranvier rely on distinct sources of proteins and targeting mechanisms
Abstract
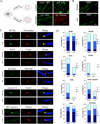
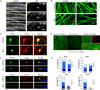
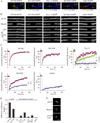
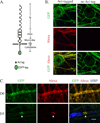
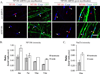
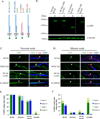
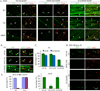
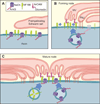
We have investigated the source(s) and targeting of components to PNS nodes of Ranvier. We show adhesion molecules are freely diffusible within the axon membrane and accumulate at forming nodes from local sources, whereas ion channels and cytoskeletal components are largely immobile and require transport to the node. We further characterize targeting of NF186, an adhesion molecule that pioneers node formation. NF186 redistributes to nascent nodes from a mobile, surface pool. Its initial accumulation and clearance from the internode require extracellular interactions, whereas targeting to mature nodes, i.e., those flanked by paranodal junctions, requires intracellular interactions. After incorporation into the node, NF186 is immobile, stable, and promotes node integrity. Thus, nodes assemble from two sources: adhesion molecules, which initiate assembly, accumulate by diffusion trapping via interactions with Schwann cells, whereas ion channels and cytoskeletal components accumulate via subsequent transport. In mature nodes, components turnover slowly and are replenished via transport.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
Comment in
-
Targeting mechanisms in myelinated axons: not all nodes are created equal.Dev Cell. 2012 Jan 17;22(1):7-9. doi: 10.1016/j.devcel.2011.12.012. Dev Cell. 2012. PMID: 22264726
References
-
- Aigner L, Arber S, Kapfhammer JP, Laux T, Schneider C, Botteri F, Brenner HR, Caroni P. Overexpression of the neural growth-associated protein GAP-43 induces nerve sprouting in the adult nervous system of transgenic mice. Cell. 1995;83:269–278. - PubMed
-
- Armstrong R, Toews AD, Morell P. Axonal transport through nodes of Ranvier. Brain Res. 1987;412:196–199. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

