Multicellular tumor spheroid model to evaluate spatio-temporal dynamics effect of chemotherapeutics: application to the gemcitabine/CHK1 inhibitor combination in pancreatic cancer
- PMID: 22244109
- PMCID: PMC3280152
- DOI: 10.1186/1471-2407-12-15
Multicellular tumor spheroid model to evaluate spatio-temporal dynamics effect of chemotherapeutics: application to the gemcitabine/CHK1 inhibitor combination in pancreatic cancer
Abstract
Background: The multicellular tumor spheroid (MCTS) is an in vitro model associating malignant-cell microenvironment and 3D organization as currently observed in avascular tumors.
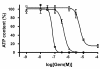
Methods: In order to evaluate the relevance of this model for pre-clinical studies of drug combinations, we analyzed the effect of gemcitabine alone and in combination with the CHIR-124 CHK1 inhibitor in a Capan-2 pancreatic cell MCTS model.
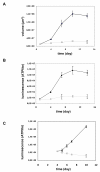
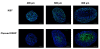
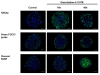
Results: Compared to monolayer cultures, Capan-2 MCTS exhibited resistance to gemcitabine cytotoxic effect. This resistance was amplified in EGF-deprived quiescent spheroid suggesting that quiescent cells are playing a role in gemcitabine multicellular resistance. After a prolonged incubation with gemcitabine, DNA damages and massive apoptosis were observed throughout the spheroid while cell cycle arrest was restricted to the outer cell layer, indicating that gemcitabine-induced apoptosis is directly correlated to DNA damages. The combination of gemcitabine and CHIR-124 in this MCTS model, enhanced the sensitivity to the gemcitabine antiproliferative effect in correlation with an increase in DNA damage and apoptosis.
Conclusions: These results demonstrate that our pancreatic MCTS model, suitable for both screening and imaging analysis, is a valuable advanced tool for evaluating the spatio-temporal effect of drugs and drug combinations in a chemoresistant and microenvironment-depending tumor model.
Figures


References
-
- Azorsa DO, Gonzales IM, Basu GD, Choudhary A, Arora S, Bisanz KM, Kiefer JA, Henderson MC, Trent JM, Von Hoff DD, Mousses S. Synthetic lethal RNAi screening identifies sensitizing targets for gemcitabine therapy in pancreatic cancer. J Transl Med. 2009;7:43. doi: 10.1186/1479-5876-7-43. - DOI - PMC - PubMed
-
- Matthews DJ, Yakes FM, Chen J, Tadano M, Bornheim L, Clary DO, Tai A, Wagner JM, Miller N, Kim YD, Robertson S, Murray L, Karnitz LM. Pharmacological abrogation of S-phase checkpoint enhances the anti-tumor activity of gemcitabine in vivo. Cell Cycle. 2007;6:104–110. doi: 10.4161/cc.6.1.3699. - DOI - PubMed
-
- Parsels LA, Morgan MA, Tanska DM, Parsels JD, Palmer BD, Booth RJ, Denny WA, Canman CE, Kraker AJ, Lawrence TS, Maybaum J. Gemcitabine sensitization by checkpoint kinase 1 inhibition correlates with inhibition of a Rad51 DNA damage response in pancreatic cancer cells. Mol Cancer Ther. 2009;8:45–54. doi: 10.1158/1535-7163.MCT-08-0662. - DOI - PMC - PubMed
-
- Morgan MA, Parsels LA, Zhao L, Parsels JD, Davis MA, Hassan MC, Arumugarajah S, Hylander-Gans L, Morosini D, Simeone DM, Canman CE, Normolle DP, Zabludoff SD, Maybaum J, Lawrence TS. Mechanism of radiosensitization by the Chk1/2 inhibitor AZD7762 involves abrogation of the G2 checkpoint and inhibition of homologous recombinational DNA repair. Cancer Res. 2010;70:4972–4981. doi: 10.1158/0008-5472.CAN-09-3573. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

