Interim results from the international trial of Second Sight's visual prosthesis
- PMID: 22244176
- PMCID: PMC3319859
- DOI: 10.1016/j.ophtha.2011.09.028
Interim results from the international trial of Second Sight's visual prosthesis
Abstract
Purpose: This study evaluated the Argus II Retinal Prosthesis System (Second Sight Medical Products, Inc., Sylmar, CA) in blind subjects with severe outer retinal degeneration.
Design: Single-arm, prospective, multicenter clinical trial.
Participants: Thirty subjects were enrolled in the United States and Europe between June 6, 2007, and August 11, 2009. All subjects were followed up for a minimum of 6 months and up to 2.7 years.
Methods: The electronic stimulator and antenna of the implant were sutured onto the sclera using an encircling silicone band. Next, a pars plana vitrectomy was performed, and the electrode array and cable were introduced into the eye via a pars plana sclerotomy. The microelectrode array then was tacked to the epiretinal surface.
Main outcome measures: The primary safety end points for the trial were the number, severity, and relation of adverse events. Principal performance end points were assessments of visual function as well as performance on orientation and mobility tasks.
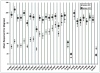
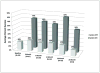
Results: Subjects performed statistically better with the system on versus off in the following tasks: object localization (96% of subjects), motion discrimination (57%), and discrimination of oriented gratings (23%). The best recorded visual acuity to date is 20/1260. Subjects' mean performance on orientation and mobility tasks was significantly better when the system was on versus off. Seventy percent of the patients did not have any serious adverse events (SAEs). The most common SAE reported was either conjunctival erosion or dehiscence over the extraocular implant and was treated successfully in all subjects except in one, who required explantation of the device without further complications.
Conclusions: The long-term safety results of Second Sight's retinal prosthesis system are acceptable, and most subjects with profound visual loss perform better on visual tasks with system than without it.
Copyright © 2012 American Academy of Ophthalmology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interest related to the study sponsor, Second Sight Medical Products (SSMP):
Mark S. Humayun has financial interests in SSMP and is employed by an institution that receives funds from SSMP
Jessy D. Dorn and Robert J. Greenberg are employed by and have stock options in SSMP
Aries Arditi has received a consulting fee from SSMP and was employed by an institution that receives funds from SSMP
Artur V. Cideciyan, Lyndon da Cruz, Gislin Dagnelie, Dean Eliott, Eugene Filley, Avinoam B. Safran, and José-Alain Sahel are or were employed by institutions that receive funds from SSMP
Jacque L.Duncan, Lucian V. Del Priore, Allen C. Ho, Arturo Santos, and Paulo E. Stanga have no conflict of interests to disclose
Figures




References
-
- MacLaren RE, Pearson RA. Stem cell therapy and the retina. Eye (Lond) 2007;21:1352–9. - PubMed
-
- Radtke ND, Aramant RB, Petry HM, et al. Vision improvement in retinal degeneration patients by implantation of retina together with retinal pigment epithelium. Am J Ophthalmol. 2008;146:172–82. - PubMed
-
- Dobelle WH, Mladejovsky MG, Girvin JP. Artifical vision for the blind: electrical stimulation of visual cortex offers hope for a functional prosthesis. Science. 1974;183:440–4. - PubMed
-
- Veraart C, Raftopoulos C, Mortimer JT, et al. Visual sensations produced by optic nerve stimulation using an implanted self-sizing spiral cuff electrode. Brain Res. 1998;813:181–6. - PubMed
-
- Humayun MS, de Juan E, Jr, Dagnelie G, et al. Visual perception elicited by electrical stimulation of retina in blind humans. Arch Ophthalmol. 1996;114:40–6. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

