Acute and prolonged hindlimb exercise elicits different gene expression in motoneurons than sensory neurons after spinal cord injury
- PMID: 22244304
- PMCID: PMC3273584
- DOI: 10.1016/j.brainres.2011.12.015
Acute and prolonged hindlimb exercise elicits different gene expression in motoneurons than sensory neurons after spinal cord injury
Abstract
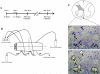
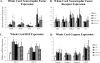
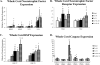
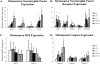
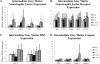
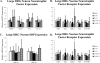
We examined gene expression in the lumbar spinal cord and the specific response of motoneurons, intermediate gray and proprioceptive sensory neurons after spinal cord injury and exercise of hindlimbs to identify potential molecular processes involved in activity dependent plasticity. Adult female rats received a low thoracic transection and passive cycling exercise for 1 or 4weeks. Gene expression analysis focused on the neurotrophic factors: brain-derived neurotrophic factor (BDNF), glial cell line-derived neurotrophic factor (GDNF), neurotrophin-3 (NT-3), neurotrophin-4 (NT-4), and their receptors because of their potential roles in neural plasticity. We also examined expression of genes involved in the cellular response to injury: heat shock proteins (HSP) -27 and -70, glial fibrillary acidic protein (GFAP) and caspases -3, -7, and -9. In lumbar cord samples, injury increased the expression of mRNA for TrkB, all three caspases and the HSPs. Acute and prolonged exercise increased expression of mRNA for the neurotrophic factors BDNF and GDNF, but not their receptors. It also increased HSP expression and decreased caspase-7 expression, with changes in protein levels complimentary to these changes in mRNA expression. Motoneurons and intermediate gray displayed little change in mRNA expression following injury, but acute and prolonged exercise increased levels of mRNA for BDNF, GDNF and NT-4. In large DRG neurons, mRNA for neurotrophic factors and their receptors were largely unaffected by either injury or exercise. However, caspase mRNA expression was increased by injury and decreased by exercise. Our results demonstrate that exercise affects expression of genes involved in plasticity and apoptosis in a cell specific manner and that these change with increased post-injury intervals and/or prolonged periods of exercise.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures
Similar articles
-
Exercise-induced gene expression in soleus muscle is dependent on time after spinal cord injury in rats.Muscle Nerve. 2004 Jan;29(1):73-81. doi: 10.1002/mus.10511. Muscle Nerve. 2004. PMID: 14694501
-
GDNF and NGF family members and receptors in human fetal and adult spinal cord and dorsal root ganglia.J Comp Neurol. 2001 Nov 12;440(2):204-17. doi: 10.1002/cne.1380. J Comp Neurol. 2001. PMID: 11745618
-
Neurotrophic factors expressed in both cortex and spinal cord induce axonal plasticity after spinal cord injury.J Neurosci Res. 2003 Oct 15;74(2):221-6. doi: 10.1002/jnr.10718. J Neurosci Res. 2003. PMID: 14515351
-
Functional recovery after cervical spinal cord injury: Role of neurotrophin and glutamatergic signaling in phrenic motoneurons.Respir Physiol Neurobiol. 2016 Jun;226:128-36. doi: 10.1016/j.resp.2015.10.009. Epub 2015 Oct 23. Respir Physiol Neurobiol. 2016. PMID: 26506253 Free PMC article. Review.
-
Role of neurotrophins in recovery of phrenic motor function following spinal cord injury.Respir Physiol Neurobiol. 2009 Nov 30;169(2):218-25. doi: 10.1016/j.resp.2009.08.008. Epub 2009 Aug 22. Respir Physiol Neurobiol. 2009. PMID: 19703592 Free PMC article. Review.
Cited by
-
The role of walking exercise on axonal regrowth and neuropathic pain markers in dorsal root ganglion after sciatic nerve injury.J Exerc Rehabil. 2023 Dec 26;19(6):320-326. doi: 10.12965/jer.2346522.261. eCollection 2023 Dec. J Exerc Rehabil. 2023. PMID: 38188130 Free PMC article.
-
Incorporating Combinatorial Approaches to Encourage Targeted Neural Stem/Progenitor Cell Integration Following Transplantation in Spinal Cord Injury.Stem Cells Transl Med. 2023 Apr 17;12(4):207-214. doi: 10.1093/stcltm/szad008. Stem Cells Transl Med. 2023. PMID: 36892546 Free PMC article. Review.
-
Benefits of exercise intervention in reducing neuropathic pain.Front Cell Neurosci. 2014 Apr 4;8:102. doi: 10.3389/fncel.2014.00102. eCollection 2014. Front Cell Neurosci. 2014. PMID: 24772065 Free PMC article. Review.
-
Cortical reorganization after spinal cord injury: always for good?Neuroscience. 2014 Dec 26;283:78-94. doi: 10.1016/j.neuroscience.2014.06.056. Epub 2014 Jul 2. Neuroscience. 2014. PMID: 24997269 Free PMC article. Review.
-
Passive cycling in neurorehabilitation after spinal cord injury: A review.J Spinal Cord Med. 2017 Jan;40(1):8-16. doi: 10.1080/10790268.2016.1248524. Epub 2016 Nov 14. J Spinal Cord Med. 2017. PMID: 27841091 Free PMC article. Review.
References
-
- Ament W, Verkerke GJ. Exercise and fatigue. Sports Med. 2009;39:389–422. DOI: 00007256-200939050-00005 [pii] - PubMed
-
- Barres BA, Raff MC, Gaese F, Bartke I, Dechant G, Barde YA. A crucial role for neurotrophin-3 in oligodendrocyte development. Nature. 1994;367:371–5. DOI: 10.1038/367371a0. - PubMed
-
- Beaumont E, Houle JD, Peterson CA, Gardiner PF. Passive exercise and fetal spinal cord transplant both help to restore motoneuronal properties after spinal cord transection in rats. Muscle Nerve. 2004;29:234–42. DOI: 10.1002/mus.10539. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

