Suppression of canine myeloid cells by soluble factors from cultured canine tumor cells
- PMID: 22244518
- PMCID: PMC4278197
- DOI: 10.1016/j.vetimm.2011.12.018
Suppression of canine myeloid cells by soluble factors from cultured canine tumor cells
Abstract
Background: Cancer profoundly affects immunity and causes immunosuppression that contributes to tumor escape, metastases and resistance to therapy. The mechanisms by which cancer cells influence immune cells are not fully known but both innate and adaptive immune cells can be altered by cancer. Myeloid cells are innate immune cells that comprise the mononuclear phagocytic system (MPS) and include monocytes, macrophages, dendritic cells (DCs) and their progenitors. Myeloid cells play important roles in both the promotion and regulation of immune responses. Dysregulated myeloid cells are increasingly being recognized as contributing to cancer-related immunosuppression. This study investigated whether soluble factors produced by canine tumor cells inhibited canine myeloid cell function.
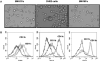
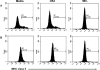
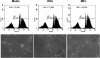
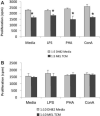
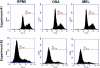
Methods: These studies investigated the utility of using the canine DH82 cell line for assessment of canine myeloid responses to tumor-derived soluble factors (TDSFs). Phenotypic comparisons to canine bone marrow-derived DCs (BM-DCs) and bone marrow-derived macrophages (BM-MΦs) were performed and expression of myeloid cell markers CD11b, CD11c, CD80, and major histocompatibility complex (MHC) class II were evaluated by flow cytometry. Phenotypic and functional changes of DC populations were then determined following exposure to tumor-conditioned media (TCM) from canine osteosarcoma, melanoma and mammary carcinoma cell lines.
Results: We found that the canine BM-DCs and the DH82 cell line shared similar CD11b, CD11c and MHC II expression and morphologic characteristics that were distinct from canine BM-MΦs. Myeloid cells exposed to TDSFs showed decreased expression of MHC class II and CD80, had reduced phagocytic activity and suppressed the proliferation of responder immune cells.
Conclusion: These results show that soluble factors secreted from canine tumor cells suppress the activation and function of canine myeloid cells. Our results suggest that, similar to humans, dysregulated myeloid cells may contribute to immunosuppression in dogs with cancer.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures
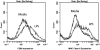
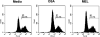
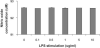

Similar articles
-
Bone marrow-derived dendritic cell vaccination of dogs with naturally occurring melanoma by using human gp100 antigen.J Vet Intern Med. 2005 Jan-Feb;19(1):56-63. doi: 10.1892/0891-6640(2005)19<56:bmdcvo>2.0.co;2. J Vet Intern Med. 2005. PMID: 15715049
-
Identification of myeloid derived suppressor cells in the peripheral blood of tumor bearing dogs.BMC Vet Res. 2012 Oct 31;8:209. doi: 10.1186/1746-6148-8-209. BMC Vet Res. 2012. PMID: 23110794 Free PMC article.
-
Modulation by canine interferon-gamma of major histocompatibility complex and tumor-associated antigen expression in canine mammary tumor and melanoma cell lines.Anticancer Res. 1995 May-Jun;15(3):923-9. Anticancer Res. 1995. PMID: 7645983
-
Comparative Immunology and Immunotherapy of Canine Osteosarcoma.Adv Exp Med Biol. 2020;1258:199-221. doi: 10.1007/978-3-030-43085-6_14. Adv Exp Med Biol. 2020. PMID: 32767244 Review.
-
Species-specific properties and translational aspects of canine dendritic cells.Vet Immunol Immunopathol. 2013 Feb 15;151(3-4):181-92. doi: 10.1016/j.vetimm.2012.12.003. Epub 2012 Dec 13. Vet Immunol Immunopathol. 2013. PMID: 23280245 Review.
Cited by
-
Canine mammary cancer cells direct macrophages toward an intermediate activation state between M1/M2.BMC Vet Res. 2015 Jul 15;11:151. doi: 10.1186/s12917-015-0473-y. BMC Vet Res. 2015. PMID: 26174804 Free PMC article.
-
DH82 Canine and RAW264.7 Murine Macrophage Cell Lines Display Distinct Activation Profiles Upon Interaction With Leishmania infantum and Leishmania amazonensis.Front Cell Infect Microbiol. 2020 Jun 12;10:247. doi: 10.3389/fcimb.2020.00247. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32596164 Free PMC article.
-
The immunotherapy of canine osteosarcoma: a historical and systematic review.J Vet Intern Med. 2015 May-Jun;29(3):759-69. doi: 10.1111/jvim.12603. Epub 2015 Apr 30. J Vet Intern Med. 2015. PMID: 25929293 Free PMC article.
-
Bacterial urinary tract infection and subclinical bacteriuria in dogs receiving antineoplastic chemotherapy.J Vet Intern Med. 2022 May;36(3):1005-1015. doi: 10.1111/jvim.16410. Epub 2022 May 7. J Vet Intern Med. 2022. PMID: 35524488 Free PMC article.
-
Inhibition of Microbicidal Activity of Canine Macrophages DH82 Cell Line by Capsular Polysaccharides from Cryptococcus neoformans.J Fungi (Basel). 2024 May 8;10(5):339. doi: 10.3390/jof10050339. J Fungi (Basel). 2024. PMID: 38786693 Free PMC article.
References
-
- Barnes A, Bee A, Bell S, Gilmore W, Mee A, Morris R, Carter SD. Immunological and inflammatory characterisation of three canine cell lines: K1, K6 and DH82. Veterinary Immunology and Immunopathology. 2000;75:9–25. - PubMed
-
- Bird RC, Deinnocentes P, Lenz S, Thacker EE, Curiel DT, Smith BF. An allogeneic hybrid-cell fusion vaccine against canine mam-mary cancer. Veterinary Immunology and Immunopathology. 2008;123:289–304. - PubMed
-
- Bonnefont-Rebeix C, de Carvalho CM, Bernaud J, Chabanne L, Marchal T, Rigal D. CD86 molecule is a specific marker for canine monocyte-derived dendritic cells. Veterinary Immunology and Immunopathology. 2006;109:167–176. - PubMed
-
- Chioda M, Peranzoni E, Desantis G, Papalini F, Falisi E, Samantha S, Mandruzzato S, Bronte V. Myeloid cell diversification and complexity: an old concept with new turns in oncology. Cancer Metastasis Reviews. 2011 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

